

硬碳材料电极首周嵌钠过程的电化学阻抗谱研究
Electrochemical Impedance Spectroscopy Study on the First Sodium Insertion Process of Hard Carbon Material Electrode
Received date: 2019-03-29
Online published: 2019-06-13
渠璐平 , 任彤 , 王宁 , 史月丽 , 庄全超 . 硬碳材料电极首周嵌钠过程的电化学阻抗谱研究[J]. 化学学报, 2019 , 77(7) : 634 -640 . DOI: 10.6023/A19030103
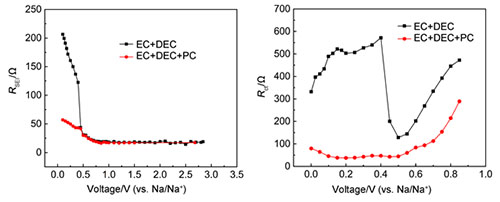
In this study, electrochemical impedance spectroscopy (EIS) combined with cyclic volt-ampere (CV), charge-discharge measurement and scanning electron microscope were used. The electrode interface characteristics of hard carbon electrodes for sodium ion batteries in 1 mol/L NaClO4-EC:DEC and 1 mol/L-NaClO4-EC:DEC:PC electrolyte systems were discussed. The hard carbon material electrode is composed of 80 wt% active material, 10 wt% PVDF-HFP adhesive and 10 wt% conductive carbon black. The charge and discharge performance was tested with 2032 button battery and metal sodium sheet as counter electrode, the charge and discharge rate was 0.1 C, and the cut-off voltage was 0~3 V. The three-electrode glass cell system was used for CV and EIS test, and the metal sodium sheet was used as the reference and auxiliary electrode. In the CV test, the scanning speed is 1 mV/s, EIS and the frequency scanning range is 105 to 10-2 Hz. The amplitude of AC signal applied by 2 mV is 5 mV. The electrochemical impedance spectra obtained in the experiment were simulated by Zview software. The results of CV show that the intercalation process of sodium ion in hard carbon materials is mainly divided into two steps, that is, the filling process of sodium ion in nano-pores, the intercalation of sodium ion in graphene layer and the adsorption and desorption of sodium ion on the surface or defect. The filling process of sodium ion in the nanoporous is accompanied by the formation of solid electrolyte interface (SEI) film on the surface of the electrode. The results of electrochemical impedance spectroscopy show that the spectrum consists of two semicircles and a oblique line, which can be attributed to the contact impedance, the diffusion of sodium ions through SEI film and the process of charge transfer. The oblique domain reflects the oblique line related to the solid diffusion of sodium ion in the particles of hard carbon materials. By selecting the appropriate equivalent circuit and fitting the experimental results, we can get the variation of SEI film resistance and electron resistance with the electrode polarization potential in the process of sodium insertion in the first week of the hard carbon electrode.
[1] Tarascon, J. M. Nat. Chem. 2010, 2, 510.
[2] Xiang, X. D.; Lu, Y. Y.; Chen, J. Acta Chim. Sinica 2017, 75, 154. (向兴德, 卢艳莹, 陈军, 化学学报, 2017, 75, 154.)
[3] Vikström, H.; Davidsson, S.; Höök, M. Appl. Energy 2013, 110, 252.
[4] Kundu, D.; Talaie, E.; Duffort, V.; Nazar, L. F. Angew. Chem., Int. Ed. 2015, 54, 3431.
[5] Li, H.; Wang, Z.; Chen, L.; Huang, X. Adv. Mater. 2009, 21, 4593.
[6] Komaba, S.; Murata, W.; Ishikawa, T.; Yabuuchi, N.; Ozeki, T.; Nakayama, T.; Ogata, A.; Gotoh, K.; Fujiwara, K. Adv. Funct. Mater. 2011, 21, 3859.
[7] Zhang, S. W.; Zhang, J.; Wu, S. D.; Lv, W.; Kang, F. Y.; Yang, Q. H. Acta Chim. Sinica 2017, 75, 163. (张思伟, 张俊, 吴思达, 吕伟, 康飞宇, 杨全红, 化学学报, 2017, 75, 163.)
[8] Wang, L.; Yang, G. R.; Wang, J. N.; Wang, S. L.; Peng, S. J.; Yan, W. Acta Chim. Sinica 2018, 76, 666. (王玲, 杨国锐, 王嘉楠, 王思岚, 彭生杰, 延卫, 化学学报, 2018, 76, 666.)
[9] Narayanrao, R.; Joglekar, M.; Inguva, S. J. Electrochem. Soc. 2013, 160, A125.
[10] Lin, X.; Park, J.; Liu, L.; Lee, Y.; Sastry, A.; Lu, W. J. Electrochem. Soc. 2013, 160, A1701.
[11] Pinson, M. B.; Bazant, M. Z. J. Electrochem. Soc. 2013, 160, A243.
[12] Xu, K. Chem. Rev. 2014, 114, 11503.
[13] Zhuang, Q. C.; Xu, S. D.; Qiu, X. Y.; Cui, Y. L.; Fang, L.; Sun, S. G. Prog. Chem. 2010, 22, 1044. (庄全超, 徐守冬, 邱祥云, 崔永丽, 方亮, 孙世刚, 化学进展, 2010, 22, 1044.)
[14] Qin, Y. P.; Zhuang, Q. C.; Shi, Y. L.; Jiang, L.; Sun, Z.; Sun, S. G. Prog. Chem. 2011, 23, 390. (秦银平, 庄全超, 史月丽, 江利, 孙智, 孙世刚, 化学进展, 2011, 23, 390.)
[15] Qiu, X. Y.; Zhuang, Q. C.; Zhang, Q. Q.; Cao, R.; Ying, P. Z.; Qiang, Y. H.; Sun, S. G. Phys. Chem. Chem. Phys. 2012, 14, 2617.
[16] Zhuang, Q. C.; Wei, T.; Du, L. L.; Cui, Y. L.; Fang, L.; Sun, S. G. J. Phys. Chem. C 2010, 114, 8614.
[17] Qiu, X. Y.; Zhuang, Q. C.; Zhang, Q. Q.; Cao, R.; Qiang, Y. H.; Ying, P. Z.; Sun, S. G. J. Electroanal. Chem. 2012, 687, 35.
[18] Wei, T.; Zhuang, Q. C.; Wu, C.; Cui, Y. L.; Fang, L.; Sun, S. G. Acta Chim. Sinica 2010, 68, 1481. (魏涛, 庄全超, 吴超, 崔永丽, 方亮, 孙世刚, 化学学报, 2010, 68, 1481.)
[19] Zhuang, Q. C.; Wei, T.; Wei, G. Z.; Dong, Q. F.; Sun, S. G. Acta Chim. Sinica 2009, 67, 2184. (庄全超, 魏涛, 魏国祯, 董全峰, 孙世刚, 化学学报, 2009, 67, 2184.)
[20] Zheng, M.; Liu, Y.; Xiao, Y.; Zhu, Y.; Guan, Q.; Yuan, D.; Zhang, J. J. Phys. Chem. C 2009, 113, 8455.
[21] Cao, Y.; Xiao, L.; Sushko, M. L.; Wang, W.; Schwenzer, B.; Xiao, J.; Nie, Z.; Saraf, L. V.; Yang, Z.; Liu, J. Nano Lett. 2012, 12, 3783.
[22] Li, Y.; Hu, Y. S.; Titirici, M. M.; Chen, L.; Huang, X. Adv. Energy Mater. 2016, 6, 1600659.
[23] Liu, P.; Li, Y.; Hu, Y. S.; Li, H.; Chen, L.; Huang, X. J. Mater. Chem. A 2016, 4, 13046.
[24] Holzapfel, M.; Martinent, A.; Alloin, F.; Le Gorrec, B.; Yazami, R.; Montella, C. J. Electroanal. Chem. 2003, 546, 41.
[25] Chang, Y. C.; Sohn, H. J. J. Electrochem. Soc. 2000, 147, 50.
[26] Levi, M.; Aurbach, D. J. Phys. Chem. B 1997, 101, 4630.
[27] Xu, S. D.; Zhuang, Q. C.; Tian, L. L.; Qin, Y. P.; Fang, L.; Sun, S. G. J. Phys. Chem. C 2011, 115, 9210.
[28] Zhuang, Q. C.; Li, J.; Tian, L. L. J. Power Sources 2013, 222, 177.
/
| 〈 |
|
〉 |