

基于混合自组装单分子层的可控分子整流
收稿日期: 2019-05-24
网络出版日期: 2019-08-13
基金资助
项目受国家自然科学基金(21773169);项目受国家自然科学基金(51633006);项目受国家自然科学基金(51733004)
Tuning Rectification Properties of Molecular Electronic Devices by Mixed Monolayer
Received date: 2019-05-24
Online published: 2019-08-13
Supported by
Project supported by the National Natural Science Foundation of China(21773169);Project supported by the National Natural Science Foundation of China(51633006);Project supported by the National Natural Science Foundation of China(51733004)
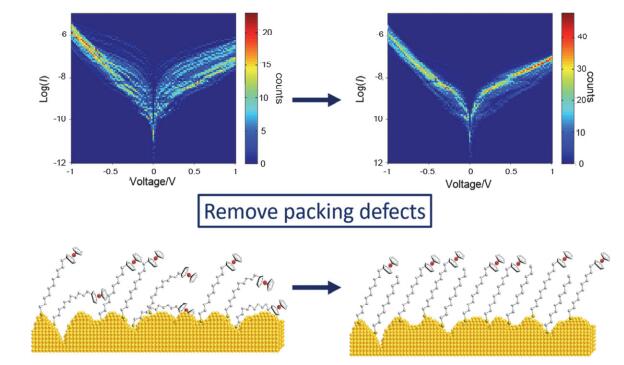
基于自组装单分子层(SAM)的分子器件的性能取决于分子层的超分子结构. 而混合自组装单分子层是一种简便易行的调控单分子层组成和结构的方法. 将具有整流特性的11-二茂铁基-1-十一烷基硫醇(FUT)和惰性1-十一烷硫醇(C11-SH)通过溶液共混制备了混合单分子层, 并用液态EGaIn作为顶电极研究了SAM器件的性质. C11-SH与FUT分子的比例可以调节器件的整流性能: 随着惰性C11-SH分子比例增高, SAM器件的整流比逐渐降低. 有趣的是, 当惰性C11-SH的比例小于20%时, 器件的整流性能反而随C11-SH的加入而得到了提升. 这是由于少量C11-SH分子减弱了由二茂铁分子的排列缺陷引起的漏电流, 改善了分子器件的稳定性和重现性, 为单分子层器件的性能调控提供了一种简便有效的方法.
王紫嫣 , Khalid,Hira , 李柏力 , 李瑶 , 于曦 , 胡文平 . 基于混合自组装单分子层的可控分子整流[J]. 化学学报, 2019 , 77(10) : 1031 -1035 . DOI: 10.6023/A19050192
We demonstrate in this work that the performance of self-assembled monolayer (SAM) molecular devices can be modulated by the composition and supramolecular structure of the molecular layer using mixed self-assembled monolayer strategy. We prepared the mixed monolayer on gold surface (with ca. 1 nm roughness) by co-adsorption of 11-(ferrocenyl)- undecanethiol (FUT, rectifier) and 1-undecanethiol (C11-SH, diluent). Micrometer scale molecular junctions were formed by using indium gallium eutectic alloy (EGaIn) as the top electrode. Electrical characterization of the junction found that the ratio of FUT and C11-SH molecules can tune the rectifying performance of the monolayer device: the higher the proportion of ferrocene is, the better the rectifying performance is. To our surprise, mixed monolayer prepared by 20% C11-SH and 80% FUT mixed solution exhibited higher rectification ratio than pure FUT monolayer, due to reduced leaking current. Surface reflective IR spectroscopy and the monolayer thickness characterization by the ellipsometer revealed loosely packed molecules on the surface in the pure FUT monolayer due to the bulky head group of the FUT and the rough gold substrate. FUT that partially lied down on the surface, or buried in the layer therefore created defects, which in turn become the origin of the leakage current. Upon insertion of C11-SH molecules in between the ferrocene molecules, the molecules in the monolayer become more ordered with the support of the C11-SH, as evidenced by decreased wave number of the C—H stretching mode of methylene group by reflective IR spectroscopy. Meanwhile, an increase in thickness for 80% FUT monolayer relative to pure FUT monolayer implied a better orientation of the FUT molecule in mixed monolayer. The ordered structure and better orientation largely improved the stability and reproducibility of the molecular device, reduced the leaking current and afforded higher rectification ratio. Our approach therefore provides a facile and effective strategy for regulating the performance of monolayer devices by molecule aggregation state.

| [1] | Jiang, L.; Huang, G. F.; Li, H. X.; Li, X. F.; Hu, W. P.; Liu, Y. Q.; Zhu, D. B . Prog. Chem. 2005, 17,, 172 (in Chinese). |
| [1] | ( 江浪, 黄桂芳, 李洪祥, 李小凡, 胡文平, 刘云圻, 朱道本, 化学进展, 2005, 17, 172.) |
| [2] | Jia, C. C.; Guo, X. F . Chem. Soc. Rev. 2013, 42, 5642. |
| [3] | Huang, C.; Rudnev, A. V.; Hong, W.; Wandlowski, T . Chem. Soc. Rev. 2015, 44, 889. |
| [4] | Li, T.; Hu, W.; Zhu, D . Adv. Mater. 2010, 22, 286. |
| [5] | Xin, N.; Guan, J.; Zhou, C.; Chen, X.; Gu, C.; Li, Y.; Ratner, M. A.; Nitzan, A.; Stoddart, J. F.; Guo, X . Nat. Rev. Phys. 2019, 1, 211. |
| [6] | Pan, Z.-C.; Li, J.; Chen, L.; Tang, Y.; Shi, J.; Liu, J.; Liao, J.-L.; Hong, W . Sci. China Chem. 2019, 62, 1245. |
| [7] | Yuan, L.; Wang, L.; Garrigues, A. R.; Jiang, L.; Annadata, H. V.; Anguera Antonana, M.; Barco, E.; Nijhuis, C. A. Nat. Nanotechnol. 2018, 13, 322. |
| [8] | Yang, Y.; Liu, J. Y.; Yan, R. W.; Wu, D. Y.; Tian, Z. Q . Chem. J. Chin. Univ. 2015, 36,, 9 (in Chinese). |
| [8] | ( 杨扬, 刘俊扬, 晏润文, 吴德印, 田中群 , 高等学校化学学报, 2015, 36, 9) |
| [9] | Xiang, D.; Wang, X.; Jia, C.; Lee, T.; Guo, X . Chem. Rev. 2016, 116, 4318. |
| [10] | Yu, P.; Feng, A.; Zhao, S.; Wei, J.; Yang, Y.; Shi, J.; Hong, W . Acta Phys.-Chim. Sin. 2019, 35,, 829 (in Chinese). |
| [10] | ( 余培锴, 冯安妮, 赵世强, 魏珺颖, 杨扬, 师佳, 洪文晶 , 物理化学学报, 2019, 35, 829. ) |
| [11] | Ai, Y.; Zhang, H . Acta Phys.-Chim. Sin. 2012, 28,, 2237 (in Chinese). |
| [11] | ( 艾勇, 张浩力 , 物理化学学报, 2012, 28, 2237. ) |
| [12] | Chen, F.; Tao, N. J . Acc. Chem. Res. 2009, 42, 429. |
| [13] | Chen, L. J.; Feng, A. N.; Wang, M. N.; Liu, J. y.; Hong, W. J.; Guo, X. F.; Xiang, D. Sci. China Chem. 2018, 61, 1368. |
| [14] | Liu, J.; Huang, X.; Wang, F.; Hong, W . Acc. Chem. Res. 2019, 52, 151. |
| [15] | Zhang, X.; Li, T . Chin. Chem. Lett. 2017, 28, 2058. |
| [16] | Han, B.; Yu, X.; Hu, W . Chem. J. Chin. Univ. 2019, 40,, 298 (in Chinese). |
| [16] | ( 韩宾, 于曦, 胡文平 , 高等学校化学学报, 2019, 40, 298. ) |
| [17] | Xu, X. N.; Han, B.; Yu, X.; Zhu, Y. Y . Acta Chim. Sinica 2019, 77,, 485 (in Chinese). |
| [17] | ( 许晓娜, 韩宾, 于曦, 朱艳英, 化学学报, 2019, 77, 485) |
| [18] | Yuan, L.; Jiang, L.; Zhang, B.; Nijhuis, C. A . Angew. Chem. Int. Ed. 2014, 53, 3377. |
| [19] | Tian, H.; Dai, Y.; Shao, H.; Yu, H.-Z . J. Phys. Chem. C 2013, 117, 1006. |
| [20] | Nerngchamnong, N.; Yuan, L.; Qi, D. C.; Li, J.; Thompson, D.; Nijhuis, C. A . Nat Nanotechnol. 2013, 8, 113. |
| [21] | Weiss, E. A.; Chiechi, R. C.; Kaufman, G. K.; Kriebel, J. K.; Li, Z.; Duati, M.; Rampi, M. A.; Whitesides, G. M . J. Am. Chem. Soc. 2017, 129, 4336. |
| [22] | Nerngchamnong, N.; Thompson, D.; Cao, L.; Yuan, L.; Jiang, L.; Roemer, M.; Nijhuis, C. A . J. Phys. Chem. C 2015, 119, 21978. |
| [23] | Yuan, L.; Jiang, L.; Thompson, D.; Nijhuis, C. A . J. Am. Chem. Soc. 2014, 136, 6554. |
| [24] | Miller, M. S.; Ferrato, M. A.; Niec, A.; Biesinger, M. C.; Carmichael, T. B . Langmuir. 2014, 30, 14171. |
| [25] | Vilan, A.; Aswal, D.; Cahen, D . Chem. Rev. 2017, 117, 4248. |
| [26] | Souto, M.; Diez-Cabanes, V.; Yuan, L.; Kyvik, A. R.; Ratera, I.; Nijhuis, C. A.; Cornil, J.; Veciana, J. Phys. Chem. Chem. Phys. 2018, 20, 25638. |
| [27] | Gao, D.; Scholz, F.; Nothofer, H. G.; Ford, W. E.; Scherf, U.; Wessels, J. M.; Yasuda, A.; von Wrochem, F. J. Am. Chem. Soc. 2011, 133, 5921. |
| [28] | Yuan, L.; Thompson, D.; Cao, L.; Nerngchangnong, N.; Nijhuis, C. A . J. Phys. Chem. C 2015, 119, 17910. |
| [29] | von Wrochem, F.; Gao, D.; Scholz, F.; Nothofer, H. G.; Nelles, G.; Wessels, J. M . Nat. Nanotechnol. 2010, 5, 618. |
| [30] | Thuo, M. M.; Reus, W. F.; Nijhuis, C. A.; Barber, J. R.; Kim, C.; Schulz, M. D.; Whitesides, G. M . J. Am. Chem. Soc. 2011, 133, 2962. |
| [31] | Song, P.; Yuan, L.; Roemer, M.; Jiang, L.; Nijhuis, C. A . J. Am. Chem. Soc. 2016, 138, 5769. |
| [32] | Tian, H.; Xiang, D.; Shao, H.; Yu, H.-Z . J. Phys. Chem. C 2014, 118, 13733. |
| [33] | Jin, J.; Kong, G. D.; Yoon, H. J . J. Phys. Chem. Lett. 2018, 9, 4578. |
| [34] | Kong, G. D.; Kim, M.; Cho, S. J.; Yoon, H. J. Angew. Chem. Int. Ed. 2016, 55, 10307. |
| [35] | Levine, I.; Weber, S. M.; Feldman, Y.; Bendikov, T.; Cohen, H.; Cahen, D.; Vilan, A . Langmuir 2012, 28, 404. |
| [36] | Chiechi, R. C.; Weiss, E. A.; Dickey, M. D.; Whitesides, G. M . Angew. Chem. Int. Ed. 2008, 47, 142. |
/
| 〈 |
|
〉 |