

基于AIE效应的多重刺激响应性聚合物纳米微球的制备及其细胞示踪应用
收稿日期: 2019-06-21
网络出版日期: 2019-08-13
基金资助
项目受国家自然科学基金(21761032);项目受国家自然科学基金(51363019);生态环境相关高分子材料教育部重点实验室开放基金(KF-18-05)
Preparation of Multi-stimulus Responsive Polymer Nanospheres Based on AIE Effect and Its Cell Tracing Application
Received date: 2019-06-21
Online published: 2019-08-13
Supported by
Project supported by the National Natural Science Foundation of China(21761032);Project supported by the National Natural Science Foundation of China(51363019);the Key Laboratory of Ecological Environment Related Polymer Materials, Ministry of Education Open Fund(KF-18-05)
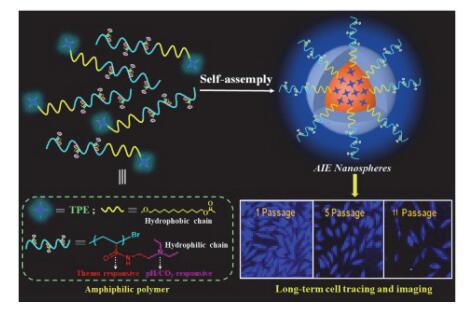
基于AIE分子和智能响应性聚合物构筑的纳米材料,具有优良的AIE发光性能、环境刺激响应性和生物相容性,已在生命科学领域展现出诱人的应用前景. 本研究通过ATRP活性聚合方法, 以合成的TPE-BIB为引发剂, 引发具有多刺激响应特性的N-[2-(二乙氨基)-乙基]丙烯酰胺单体聚合, 成功制备具有温度/pH/CO2三重响应性的两亲性聚合物: TPE-g-PDEAEAM, 并自组装形成约200 nm的纳米微球. 研究表明: 这种聚合物纳米粒子具有优良的水溶性、单分散性、稳定性及优异的AIE发光特性. 其相转变温度为60 ℃, 溶液荧光对环境温度、pH及CO2均表现出快速敏感响应性能. 同时, 该纳米粒子表现出低细胞毒性, 能够有效示踪HeLa细胞增殖至11代以上, 有望作为一种活细胞荧光示踪探针材料.
关晓琳 , 王林 , 李志飞 , 刘美娜 , 王凯龙 , 林斌 , 杨学琴 , 来守军 , 雷自强 . 基于AIE效应的多重刺激响应性聚合物纳米微球的制备及其细胞示踪应用[J]. 化学学报, 2019 , 77(10) : 1036 -1044 . DOI: 10.6023/A19060226
In recent years, fluorescent bioimaging technology has great advantages in the fields of life science research and medical diagnosis because of its advantages of fast and effective, high sensitivity, easy realization of multi-channel imaging and economic efficiency. Organic fluorescent dyes have been widely used as biological imaging reagents due to their excellent photoelectric properties, functional modification, adjustable optical properties, and good biocompatibility. However, conventional organic fluorescent molecules cause aggregation-caused quenching (ACQ) due to π-π stacking in the aggregated state, limiting their bioimaging applications in aggregated or high concentrations. Since the discovery of the unique luminescence phenomenon of aggregation-induced emission (AIE), the ACQ phenomenon of traditional fluorescent materials has been eliminated. Stimulating responsive polymer nanoparticles have been widely used in the life sciences due to their combination of nanoparticle and polymer advantages and their ability to respond intelligently with environmental changes. Therefore, nanomaterials with excellent aggregation-induced emission (AIE) property, environmental stimuli responsiveness and biocompatibility based on AIE molecules and smart responsive polymers have shown attractive application prospects in the life sciences. A kind of multi-responsive AIE-active polymer nanospheres, which were composed of tetraphenylethylene (TPE) and stimuli-responsive poly[N]-2-(diethylamino)-ethyl]acrylamide (PDEAEAM), were constructed in this study. Firstly, a multi-stimulation responsive monomer N-[2-(diethylamino)ethyl]acrylamide (DEAEAM) and TPE derivative tetraphenylethene-4-(12-hydroxydodecyl-2-methylpropionyl) (TPE-BIB) with propionyl bromide were synthesized, respectively, and a multi-stimuli-responsive amphiphilic polymer of tetraphenylethene-graft-poly[N-[2-(diethylamino)ethyl]acrylamide] (TPE-g-PDEAEAM) was then successfully synthesized by atom transfer radical polymerization (ATRP) using TPE-BIB as initiator. Lastly, polymer nanospheres TPE-g-PDEAEAM of approximately 200 nm were formed by a self-assembling pro-cess. The results of the performed experiments showed that the LCST of TPE-g-PDEAEAM in aqueous solution is about 60 ℃. Meanwhile, the luminescence change of TPE-g-PDEAEAM at different temperatures from 20 to 66 ℃ was observed. The fluorescence intensity of TPE-g-PDEAEAM firstly decreased with increasing temperature from 20 to 58 ℃, and the fluorescence intensity increased with increasing temperature from 58 to 66 ℃. The phase transfer of PDEAEAM in TPE-g-PDEAEAM may be the reason of luminescence change which may lead to the fluorescent temperature response. Moreover, the fluorescence intensity of TPE-g-PDEAEAM nanospheres in aqueous solution increased with increasing temperature pH. Besides, the fluorescence intensity of TPE-g-PDEAEAM decreased dramatically when the volume of CO2 increased from 0.0 to 1.2 mL. Therefore, TPE-g-PDEAEAM was a new temperature and pH/CO2 responsive materials and might be used as multi-functional smart fluorescent sensors. More importantly, the fluorescent signals were significantly strong in HeLa cells after cells were incubated with TPE-g-PDEAEAM for 24 h based on the characteristic of AIE fluorescence and low cytotoxicity. The resultant nanospheres were able to be internalized by the cancer cells and effectively track the HeLa cells for as long as 11 passages. So, the polymer nanomaterial is an ideal living cell fluorescent tracer probe, which is expected to be applied as biosensors, long-term cell traces and medical biomaterials.

| [1] | Xia, Z. Q.; Shao, A. D.; Li, Q.; Zhu, S. Q.; Zhu, W. H . Acta Chim. Sinica 2016, 74,, 351. |
| [1] | ( 夏志清, 邵安东, 李强, 朱世琴, 朱为宏, 化学学报, 2016, 74, 351.) |
| [2] | Guo, S.; Zheng, F.; Zeng, F.; Wu, S. Z. Chinese J. Polym. Sci. 2016, 34, 830. |
| [3] | Chen, Y.; Qiu, T.; Zhao, W.; Fan, L.. Polym. Chem . 2015, 6, 1576. |
| [4] | Yu, C.; Li, X.; Zeng, F.; Zheng, F.; Wu, S. Z. Chem. Commun. 2013, 49, 403. |
| [5] | Sun, J. B.; Zhang, G. H.; Jia, X. Y.; Xue, P. C.; Jia, J. H.; Lu, R. Acta Chim. Sinica 2016, 74, 165. |
| [5] | ( 孙静波, 张恭贺, 贾小宇, 薛鹏冲, 贾俊辉, 卢然, 化学学报, 2016, 74, 165. ) |
| [6] | Xu, S. Y.; Sun, X.; Ge, H.; Arrowsmith, R. L.; Fossey, J. S.; Pascu, S. I.; Jiang, Y. B.; James, T. D. Org. Biomol. Chem. 2015, 13, 4143. |
| [7] | Mei, J.; Leung, N. L.; Kwok, R. T.; Lam, J. W.; Tang, B. Z. Chem. Rev. 2015, 115, 11718. |
| [8] | Gao, M.; Hu, Q.; Feng, G.; Tang, B. Z.; Liu, B . J. Mater. Chem. B 2014, 2, 3438. |
| [9] | Liu, Z.; Xue, W.; Cai, Z.; Zhang, G.; Zhang, D. J. Mater. Chem. 2011, 21, 14487. |
| [10] | Tang, X.; Bai, Q.; Peng, Q.; Gao, Y.; Li, J.; Liu, Y.; Yao, L.; Lu, P.; Yang, B.; Ma, Y. . Chem Mater . 2015, 27, 7050. |
| [11] | Zhang, Y.; Kong, L.; Shi, J.; Tong, B.; Zhi, J.; Feng, X.; Dong, Y. Chin. J. Chem. 2015, 33, 701. |
| [12] | Hu, F.; Zhang, G.; Zhan, C.; Zhang, W.; Yan, Y.; Zhao, Y.; Fu, H.; Zhang, D . Small 2015, 11, 1335. |
| [13] | Hu, R.; Xin, D. H.; Qin, A. J.; Tang, B. Z . Acta Polymerica Sinica 2018, 2,, 132. |
| [13] | ( 胡蓉, 辛德华, 秦安军, 唐本忠 , 高分子学报, 2018, 2, 132. ) |
| [14] | Jiang, M. J.; Guo, Z. J.; Tang, B. Z. Sci. Technol. Rev. 2018, 36, 27. |
| [14] | ( 江美娟, 郭子健, 唐本忠, 科技导报, 2018, 36, 27. ) |
| [15] | Gao, Y.; Qu, Y.; Jiang, T.; Zhang, H.; He, N.; Li, B.; Wu, J.; Hua, J. J. Mater. Chem. C 2014, 2, 6353. |
| [16] | Li, S.; Langenegger, S. M.; H?ner, R. Chem. Commun . 2013, 49, 5835. |
| [17] | Singh, A.; Lim, C. K.; Lee, Y. D.; Maeng, J. H.; Lee, S.; Koh, J.; Kim, S. ACS Appl. Mater. Interfaces 2013, 5, 8881. |
| [18] | Ma, S. Q.; Ma, L.; Han, W. K.; Jiang, S.; Xu, B.; Tian, W. J. Sci. China Chem. 2018, 48, 683. |
| [18] | ( 马愫倩, 马莲, 韩文坤, 姜姗, 徐斌, 田文晶, 中国科学, 化学, 2018, 48, 683. ) |
| [19] | Wang, Z. L.; Yang, J. L.; Yang, Y. Q.; Xu, X.; Li, M. X.; Zhang, Y.; Fang, H.; Xu, H. J.; Wang, S. F. Chin. J. Org. Chem. 2018, 38, 1401. |
| [19] | ( 王忠龙, 杨金来, 杨益琴, 徐徐, 李明新, 张燕, 方华, 徐海军, 王石发, 有机化学, 2018, 38, 1401. ) |
| [20] | Wang, Z.; Chen, S.; Lam, J. W. Y. J. Am. Chem. Soc. 2013, 135, 8238. |
| [21] | Wang, D.; Su, H. F.; Tang, B. Z. Chem. Sci. 2018, 9, 3685. |
| [22] | Wang, J.; Wu, Y. L.; Sun, L. H.; Zeng, F.; Wu, S. Z. Acta Chim. Sinica 2016, 74, 910. |
| [22] | ( 王俊, 武英龙, 孙立和, 曾钫, 吴水珠, 化学学报, 2016, 74, 910. ) |
| [23] | Chen, S.; Jiang, F. J.; Cao, Z. Q.; Wang, G. J.; Dang, Z. M. Chem. Commun. 2015, 51, 12633. |
| [24] | Guo, J.; Wang, N. J.; Peng, L.; Wu, J. J.; Ye, Q. Q.; Yuan, J. Y. J. Mater. Chem. B 2016, 4, 4009. |
| [25] | Yuan, T. T.; Dong, J.; Hana, G. X.; Wang, G. J. RSC Adv. 2016, 6, 10904. |
| [26] | Karimi, M.; Ghasemi, A.; Zangabad, P. S.; Rahighi, R.; Beyzavi, J. A.; Vaseghi, K. A.; Haghani, M. L.; Bahramia, N. S.; Hamblin, M. R. Chem. Soc. Rev. 2016, 45, 1457. |
| [27] | Guan, X. L; Meng, L.; Jin, Q. J.; Lu, B. C.; Chen, Y. B.; Li, Z. F.; Wang, L.; Lai, S. J.; Lei, Z. Q. Macromol. Mater. Eng. 2018, 303, 1700553. |
| [28] | Wang, Z.; Yong, T. Y.; Wan, J.; Li, Z. H.; Zhao, H.; Zhao, Y.; Gan, L.; Yang, X. L.; Xu, H. B.; Zhang, C. ACS Appl. Mater. Interfaces 2015, 7, 3420. |
| [29] | Yuan, Y.; Kwok, R. T. K.; Tang, B. Z.; Liu, B. Small 2015, 11, 4682. |
| [30] | Song, Z.; Wang, K.; Gao, C.; Wang, S.; Zhang, W. Q. Macromolecules 2016, 49, 162. |
| [31] | Jiang, X.; Feng, C.; Lu, G.; Huang, X. ACS Macro Lett. 2014, 3, 1121. |
| [32] | Zhang, G. Z.; Jiang, M.; Wu, Q. Chin. Polym. Bull. 2005, 4, 82. |
| [32] | ( 张广照, 江明, 吴奇 , 高分子通报, 2005, 4, 82. ) |
| [33] | Anirudhan, T. S.; Nair, A. S. J. Mater. Chem. B 2018, 6, 428. |
| [34] | Zhang, Y. F.; Wu, T.; Liu, S. Y. Macromol. Chem. Phys. 2007, 208, 2492. |
/
| 〈 |
|
〉 |