

酶法催化二氧化碳制备高附加值化学品研究进展
收稿日期: 2019-07-01
网络出版日期: 2019-08-23
基金资助
国家自然科学基金(21676104);国家自然科学基金(21878105);国家重点研发计划(2018YFC1603400);国家重点研发计划(2018YFC1602100);广州市科技计划项目(201904010360)
Recent Advances in Enzymatic Catalysis for Preparation of High Value-Added Chemicals from Carbon Dioxide
Received date: 2019-07-01
Online published: 2019-08-23
Supported by
the National Natural Science Foundation of China(21676104);the National Natural Science Foundation of China(21878105);the National Key R&D Program of China(2018YFC1603400);the National Key R&D Program of China(2018YFC1602100);the Science and Technology Program of Guangzhou(201904010360)
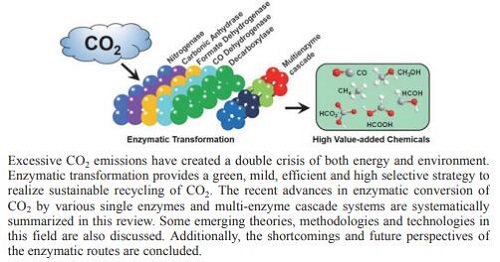
现代工业的发展不断消耗煤、石油、天然气等碳化石燃料,并产生了大量的温室气体CO2,使人类面临能源和环境的双重挑战,开发绿色能源、控制CO2对环境的影响迫在眉睫.CO2是一种廉价的碳源,可通过化学法、光化学法、电化学法或酶法等转化为高附加值含碳化学品,实现CO2的资源化循环利用,是解决全球碳排放所带来的能源和环境危机的双赢策略.受CO2胞内天然生物转化的启发,酶法为CO2的循环利用带来了新的机遇,相比于传统的化学及光、电化学法,可表现出绿色、高效、温和、高选择性等优点,有望为CO2高值化利用带来新的契机.有鉴于此,本文从胞内CO2生物转化机理出发,综述了国内外近年来多种单酶及多酶级联催化CO2高值化利用的最新研究进展,并交叉论述了固定化酶催化体系的构建、酶定向进化和改造、酶催化过程调控等内容,总结了酶法转化目前存在的缺陷和不足,并提出了展望,以期为酶法催化CO2高值化利用提供一定的参考和借鉴.
梁珊 , 宗敏华 , 娄文勇 . 酶法催化二氧化碳制备高附加值化学品研究进展[J]. 化学学报, 2019 , 77(11) : 1099 -1114 . DOI: 10.6023/A19060240
With the rapid development of modern industry, coal, petroleum, natural gas and other fossil fuels have been excessively consumed, along with an increasing large quantities of greenhouse gases (e.g. carbon dioxide, CO2) are produced. It is urgent to develop sustainable green energy and abate the detriment of carbon dioxide on global environment. CO2 is a cheap carbon source that can be converted into high value-added chemicals by chemical, photochemical, electrochemical or enzymatic methods to realize the recycling of CO2. It is a win-win strategy to solve the energy and environmental crisis caused by global carbon emissions. Inspired by natural CO2 metabolic process, enzymatic transformation provides an alternative strategy for efficient recycling of CO2. Compared with traditional chemical, photochemical or electrochemical methods, the enzymatic route holds advantages of green, high efficiency, mild and excellent selectivity, which is expected to bring new revolutionary opportunities for efficient utilization of CO2. Thus, in this present review, we firstly introduce the brief background about enzymatic conversion for CO2 capture, sequestration and utilization. Next, we depict six major routes of the CO2 metabolic process in cells, which are taken as the inspiration source for the construction of enzymatic systems in vitro. Subsequently, recent advances in enzymatic conversion of CO2 that catalyzed by various single enzymes and multi-enzyme cascade systems are systematically reviewed. Some emerging approaches for construction of immobilized single-or multi-enzyme systems, directed evolution and artificial modification of enzymes, and cofactor regulation during the enzymatic processes are also discussed. Finally, the defects and shortcomings of enzymatic approaches are summarized, and the future perspectives are finally put forward. Based on this present review, we aim to provide theoretical reference and practical basis for more efficient enzymatic utilization of CO2 to produce high value-added chemicals.

Key words: CO2; enzymatic conversion; high value conversion; reduction; cascade catalysis; energy; environment
| [1] | Air Products and Chemicals, Inc., Carbon Dioxide Product Stewardship Summary, www.airproducts.com, 2018. |
| [2] | Lei Z.; Xue Y.; Chen W.; Qiu W.; Zhang Y.; Horike S.; Tang L. Adv. Energy Mater. 2018, 8, 1801587. |
| [3] | Zhang Z.; Muschiol J.; Huang Y.; Sigurdardóttir S. B.; von Solms N.; Daugaard A. E.; Wei J.; Luo J.; Xu B.-H.; Zhang S.; Pinelo M. Green Chem. 2018, 20, 4339. |
| [4] | Sultana S.; Chandra Sahoo P.; Martha S.; Parida K. RSC Adv. 2016, 6, 44170. |
| [5] | Chang S.-L.; Liang F.; Yao Y.-C.; Ma W.-H.; Yang B.; Dai Y.-N. Acta Chim. Sinica 2018, 76, 515. |
| [5] | 常 世磊; 梁 风; 姚 耀春; 马 文会; 杨 斌; 戴 永年 化学学报 2018, 76, 515. |
| [6] | Olivier J. G. J.; Peters J. A. H. W. Trends in global CO2 and total greenhouse gas emissions:2018 Report, PBL Netherlands Environmental Assessment Agency, The Hague 2018, 3125, pp. 4 6 |
| [7] | Shi J.; Jiang Y.; Jiang Z.; Wang X.; Wang X.; Zhang S.; Han P.; Yang C. Chem. Soc. Rev. 2015, 44, 5981. |
| [8] | Zhang S.; Li X.-D.; He L.-N. Acta Chim. Sinica 2016, 74, 17 |
| [8] | 张 帅; 李 雪冬; 何 良年 化学学报 2016, 74, 17 |
| [9] | Long N.; Lee J.; Koo K.-K.; Luis P.; Lee M. Energies 2017, 10, 473. |
| [10] | Chang X.; Wang T.; Yang P.; Zhang G.; Gong J. Adv. Mater. 2018, 31, 1804710 |
| [11] | Chen Z.; Wang X.; Liu L. Chem. Rec. 2019, 19, 1272. |
| [12] | Kuramochi Y.; Ishitani O.; Ishida H. Coord. Chem. Rev. 2018, 373, 333. |
| [13] | Aresta, M.; Dibenedetto, A.; Quaranta, E. In Reaction Mechanisms in Carbon Dioxide Conversion, Vol. 9, Eds.: Aresta, M.; Dibenedetto, A.; Quaranta, E., Springer Berlin Heidelberg, Berlin, Heidelberg, 2016, pp. 347~371. |
| [14] | Mondal B.; Song J.; Neese F.; Ye S. Curr. Opin. Chem. Biol. 2015, 25, 103. |
| [15] | Aresta M.; Quaranta E.; Liberio R.; Dileo C.; Tommasi I. Tetrahedron 1998, 54, 8841. |
| [16] | Obert R.; Dave B. C. J. Am. Chem. Soc. 1999, 121, 12192. |
| [17] | Marpani F.; Pinelo M.; Meyer A. S. Biochem. Eng. J. 2017, 127, 217. |
| [18] | Fuchs G. Annu. Rev. Microbiol. 2011, 65, 631. |
| [19] | Berg, I. A.; Kockelkorn, D.; Ramos-Vera, W. H.; Say, R.; Zarzycki, J.; Fuchs, G. In Carbon Dioxide as Chemical Feedstock, Vol. 3, Ed.: Aresta, M., WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, 2010, pp. 33~53. |
| [20] | Alissandratos A.; Easton C. J. Beilstein J. Org. Chem. 2015, 11, 2370. |
| [21] | Erb T. J. Appl. Environ. Microbiol. 2011, 77, 8466. |
| [22] | Tabita F. R.; Hanson T. E.; Li H.; Satagopan S.; Singh J.; Chan S. Microbiol. Mol. Biol. Rev. 2007, 71, 576. |
| [23] | Ljungdahl L. G.; Wood H. G. Annu. Rev. Microbiol. 1969, 23, 515. |
| [24] | Ragsdale, S. W.; Kumar, M.; Seravalli, J.; Qiu, D.; Spiro, T. D. In Microbial Growth on C1 Compounds, Eds.: Lidstrom, M. E.; Tabita, F. R., Springer, Dordrecht, 1996, pp. 191~196. |
| [25] | Ragsdale S. W.; Pierce E. Biochim. Biophys. Acta 2008, 1784, 1873. |
| [26] | Gencic S.; Duin E. C.; Grahame D. A. J. Biol. Chem. 2010, 285, 15450. |
| [27] | Wang H.-J.; Ni J.; Zhang Y.; Zhang L.; Xin Y.-Y. Microbiol. China 2013, 40, 304 |
| [27] | 王 洪杰; 倪 俊; 张 怡; 张 玲; 辛 越勇 微生物学报 2013, 40, 304 |
| [28] | Scherf U.; Buckel W. Eur. J. Biochem. 1993, 215, 421. |
| [29] | Berg I. A.; Kockelkorn D.; Buckel W.; Fuchs G. Science 2007, 318, 1782. |
| [30] | Ishii M.; Miyake T.; Satoh T.; Sugiyama H.; Oshima Y.; Kodama T.; Igarashi Y. Arch. Microbiol. 1996, 166, 368. |
| [31] | Zarzycki J.; Brecht V.; Müller M.; Fuchs G. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 21317. |
| [32] | Patel H. M.; Kraszewski J. L.; Mukhopadhyay B. J. Bacteriol. 2004, 186, 5129. |
| [33] | Burgess B. K.; Lowe D. J. Chem. Rev. 1996, 96, 2983. |
| [34] | Shah V. K.; Brill W. J. Proc. Natl. Acad. Sci. U. S.A. 1977, 74, 3249. |
| [35] | Yang, Z.-Y.; Danyal, K.; Seefeldt, L. C. In Nitrogen Fixation. Methods in Molecular Biology (Methods and Protocols), Vol. 766, Ed.: Ribbe, M. W., Humana Press, Heidelberg, 2011, pp. 9~29. |
| [36] | Rivera-Ortiz J. M.; Burris R. H. J. Bacteriol. 1975, 123, 537 |
| [37] | Lee C. C.; Hu Y.; Ribbe M. W. Science 2010, 329, 642. |
| [38] | Hu Y.; Lee C. C.; Ribbe M. W. Science 2011, 333, 753. |
| [39] | Yang Z.-Y.; Moure V. R.; Dean D. R.; Seefeldt L. C. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 19644. |
| [40] | Seefeldt L. C.; Rasche M. E.; Ensign S. A. Biochemistry 1995, 34, 5382. |
| [41] | Zheng Y.; Harris D. F.; Yu Z.; Fu Y.; Poudel S.; Ledbetter R. N.; Fixen K. R.; Yang Z.-Y.; Boyd E. S.; Lidstrom M. E.; Seefeldt L. C.; Harwood C. S. Nat. Microbiol. 2018, 3, 281. |
| [42] | Rebelein J. G.; Stiebritz M. T.; Lee C. C.; Hu Y. Nat. Chem. Biol. 2016, 13, 147 |
| [43] | Khadka N.; Dean D. R.; Smith D.; Hoffman B. M.; Raugei S.; Seefeldt L. C. Inorg. Chem. 2016, 55, 8321. |
| [44] | Lindskog S. Inorg. Chim. Acta 1983, 79, 36 |
| [45] | Savile C. K.; Lalonde J. J. Curr. Opin. Biotechnol. 2011, 22, 818. |
| [46] | Smith K. S.; Jakubzick C.; Whittam T. S.; Ferry J. G. Proc. Natl. Acad. Sci. U. S. A. 1999, 96, 15184. |
| [47] | Cai L.-X.; Chu Y.-M.; Zhang G.-Y. Chin. J. Biotech. 2019, 31, 1 |
| [47] | 蔡 丽希; 楚 云猛; 张 光亚 生物工程学报 2019, 31, 1 |
| [48] | Lehtonen J.; Shen B.; Vihinen M.; Casini A.; Scozzafava A.; Supuran C.; Parkkila A.-K.; Saarnio J.; Kivel A. J.; Waheed A.; Sly W.; Parkkila S. J. Biol. Chem. 2004, 279, 2719. |
| [49] | Loferer M. J.; Tautermann C. S.; Loeffler H. H.; Liedl K. R. J. Am. Chem. Soc. 2003, 125, 8921. |
| [50] | Shekh A.; Kannan K.; Mudliar N. S.; Yadav R.; Fulke A.; Sivanesan S. d.; Chakrabarti T. Crit. Rev. Environ. Sci. Technol. 2011, 42, 1419 |
| [51] | Bond G. M.; Stringer J.; Brandvold D. K.; Simsek F. A.; Medina M.-G.; Egeland G. Energy Fuels 2001, 15, 309. |
| [52] | Yadav R. R.; Krishnamurthi K.; Mudliar S. N.; Devi S. S.; Naoghare P. K.; Bafana A.; Chakrabarti T. J. Basic Microbiol. 2014, 54, 472. |
| [53] | Mirjafari P.; Asghari K.; Mahinpey N. Ind. Eng. Chem. Res. 2007, 46, 921. |
| [54] | Xiao L.; Lian B. Carbonates Evaporites 2016, 31, 39. |
| [55] | McQ Gould S.; Tawfik D. Biochemistry 2005, 44, 5444. |
| [56] | Alvizo O.; Nguyen L. J.; Savile C. K.; Bresson J. A.; Lakhapatri S. L.; Solis E. O. P.; Fox R. J.; Broering J. M.; Benoit M. R.; Zimmerman S. A.; Novick S. J.; Liang J.; Lalonde J. J. Proc. Natl. Acad. Sci. U. S. A. 2014, 111, 16436. |
| [57] | Yoshimoto M.; Walde P. World J. Microbiol. Biotechnol. 2018, 34, 151. |
| [58] | Liu W.-F.; Wei L.-N. J. Mol. Catal. 2016, 30, 182 |
| [58] | 刘 文芳; 魏 利娜 分子催化 2016, 30, 182 |
| [59] | Yoshimoto M.; Schweizer T.; Rathlef M.; Pleij T.; Walde P. ACS Omega 2018, 3, 10391. |
| [60] | Maeshima K.; Yoshimoto M. Enzyme Microb. Technol. 2017, 105, 9. |
| [61] | Cui J.-D.; Li Y.; Ji X.-Y.; Bian H.-J.; Zhang Y.-F.; Su Z.-G.; Ma G.-H.; Zhang S.-P. Chem. J. Chin. Univ. 2014, 35, 1999. |
| [61] | 崔 建东; 李 莹; 姬 晓元; 边 红杰; 张 羽飞; 苏 志国; 马 光辉; 张 松平 高等学校化学学报 2014, 35, 1999. |
| [62] | Bulushev D. A.; Ross J. R. H. ChemSusChem 2018, 11, 821. |
| [63] | Kawanami H.; Himeda Y.; Laurenczy G. Adv. Inorg. Chem. 2017, 70, 395. |
| [64] | Castillo R.; Oliva M.; Martí S.; Moliner V. J. Phys. Chem. B 2008, 112, 10012. |
| [65] | Neuhauser W.; Steininger M.; Haltrich D.; Kulbe K. D.; Nidetzky B. Biotechnol. Bioeng. 1998, 60, 277. |
| [66] | Dong G.; Ryde U. J. Biol. Inorg. Chem. 2018, 23, 1243. |
| [67] | Boyington J. C.; Gladyshev V. N.; Khangulov S. V.; Stadtman T. C.; Sun P. D. Science 1997, 275, 1305. |
| [68] | Schrapers P.; Hartmann T.; Kositzki R.; Dau H.; Reschke S.; Schulzke C.; Leimkühler S.; Haumann M. Inorg. Chem. 2015, 54, 3260. |
| [69] | Mota C. S.; Rivas M. G.; Brondino C. D.; Moura I.; Moura J. J. G.; González P. J.; Cerqueira N. M. F. S. A. J. Biol. Inorg. Chem. 2011, 16, 1255. |
| [70] | Dobbek H. Coord. Chem. Rev. 2011, 255, 1104. |
| [71] | Parkinson B. A.; Weaver P. F. Nature 1984, 309, 148. |
| [72] | Lu Y.; Jiang Z.-Y.; Xu S.-W.; Wu H. Catal. Today 2006, 115, 263. |
| [73] | Yadav R. K.; Baeg J.-O.; Oh G. H.; Park N.-J.; Kong K.-j.; Kim J.; Hwang D. W.; Biswas S. K. J. Am. Chem. Soc. 2012, 134, 11455. |
| [74] | Choe H.; Joo J. C.; Cho D. H.; Kim M. H.; Lee S. H.; Jung K. D.; Kim Y. H. PLoS One 2014, 9, e103111. |
| [75] | Yadav R. K.; Baeg J.-O.; Kumar A.; Kong K.-j.; Oh G. H.; Park N.-J. J. Mater. Chem. A 2014, 2, 5068. |
| [76] | Woolerton T. W.; Sheard S.; Reisner E.; Pierce E.; Ragsdale S. W.; Armstrong F. A. J. Am. Chem. Soc. 2010, 132, 2132. |
| [77] | Xu C.-Y.; Lin J.-Y.; Pan F.-Q.; Den B.-W.; Wang Z.-H.; Zhou J.-H.; Chen Y.; Ma J.-C.; Gu Z.-E.; Zhang Y.-W. Acta Chim. Sinica 2017, 75, 699. |
| [77] | 许 辰宇; 林 伽毅; 潘 富强; 邓 博文; 王 智化; 周 俊虎; 陈 云; 马 京程; 顾 志恩; 张 彦威 化学学报 2017, 75, 699. |
| [78] | Jeoung, J.-H.; Martins, B. M.; Dobbek, H. In Metalloproteins: Methods and Protocols, Vol. 3, Ed.: Hu, Y., Springer, New York, 2019, pp. 37~54. |
| [79] | Appel A. M.; Bercaw J. E.; Bocarsly A. B.; Dobbek H.; DuBois D. L.; Dupuis M.; Ferry J. G.; Fujita E.; Hille R.; Kenis P. J. A.; Kerfeld C. A.; Morris R. H.; Peden C. H. F.; Portis A. R.; Ragsdale S. W.; Rauchfuss T. B.; Reek J. N. H.; Seefeldt L. C.; Thauer R. K.; Waldrop G. L. Chem. Rev. 2013, 113, 6621. |
| [80] | Parkin A.; Seravalli J.; Vincent K. A.; Ragsdale S. W.; Armstrong F. A. J. Am. Chem. Soc. 2007, 129, 10328. |
| [81] | Jeoung J.-H.; Dobbek H. Science 2007, 318, 1461. |
| [82] | Miyazaki S.; Koga Y.; Matsumoto T.; Matsubara K. Chem. Commun. 2010, 46, 1932. |
| [83] | Wu Y.-Z.; Shi J.-F.; Ding F.; Zhao J.-J.; Zou X.-Y.; Wang M.-R.; Zhang S.-H.; Tong Z.-W.; Zhang S.-P.; Jiang Z.-Y. Sci. Sin. Chim. 2017, 47, 315 |
| [83] | 伍 一洲; 石 家福; 丁 菲; 赵 晶晶; 邹 晓燕; 王 曼茹; 张 少华; 佟 振伟; 张 松平; 姜 忠义 中国科学:化学 2017, 47, 315 |
| [84] | Shin W.; Lee S. H.; Shin J. W.; Lee S. P.; Kim Y. J. Am. Chem. Soc. 2003, 125, 14688. |
| [85] | Glueck S. M.; Gümüs S.; Fabian W. M. F.; Faber K. Chem. Soc. Rev. 2010, 39, 313. |
| [86] | Allen J. R.; Ensign S. A. J. Bacteriol. 1996, 178, 1469. |
| [87] | Boll M.; Fuchs G. Biol. Chem. 2005, 386, 989. |
| [88] | Huang J.; He Z.; Wiegel J. J. Bacteriol. 1999, 181, 5119 |
| [89] | Wieser M.; Yoshida T.; Nagasawa T. J. Mol. Catal. B:Enzym. 2001, 11, 179. |
| [90] | Miyazaki M.; Shibue M.; Ogino K.; Nakamura H.; Maeda H. Chem. Commun. 2001, 18, 1800 |
| [91] | Tong X.; El-Zahab B.; Zhao X.; Liu Y.; Wang P. Biotechnol. Bioeng. 2011, 108, 465. |
| [92] | Liu W.-F.; Hou Y.-H.; Hou B.-X.; Zhao Z.-P. Chinese J. Chem. Eng. 2014, 22, 1328 |
| [92] | 刘 文芳; 侯 延慧; 侯 本象; 赵 之平 中国化学工程学报 2014, 22, 1328 |
| [93] | Liu W.-F.; Hou B.-X.; Hou Y.-H.; Zhao Z.-P. Chinese J. Catal. 2012, 33, 730 |
| [93] | 刘 文芳; 侯 本象; 侯 延慧; 赵 之平 催化学报 2012, 33, 730 |
| [94] | Li R.; Wang Z.; Ni P.; Zhao Y.; Li M.; Li L. Fuel 2014, 128, 180. |
| [95] | Jadhav S. G.; Vaidya P. D.; Bhanage B. M.; Joshi J. B. Chem. Eng. Res. Des. 2014, 92, 2557. |
| [96] | Goeppert A.; Czaun M.; Jones J.-P.; Surya Prakash G. K.; Olah G. A. Chem. Soc. Rev. 2014, 43, 7995. |
| [97] | Wang W.-H.; Himeda Y.; Muckerman J. T.; Manbeck G. F.; Fujita E. Chem. Rev. 2015, 115, 12936. |
| [98] | Kuwabata S.; Tsuda R.; Yoneyama H. J. Am. Chem. Soc. 1994, 116, 5437. |
| [99] | Baskaya F. S.; Zhao X.; Flickinger M. C.; Wang P. Appl. Biochem. Biotechnol. 2010, 162, 391. |
| [100] | Xu S.-W.; Lu Y.; Li J.; Jiang Z.-Y.; Wu H. Ind. Eng. Chem. Res. 2006, 45, 4567. |
| [101] | Sun Q.; Jiang Y.; Jiang Z.; Zhang L.; Sun X.; Li J. Ind. Eng. Chem. Res. 2009, 48, 4210. |
| [102] | Jiang Y.; Sun Q.; Zhang L.; Jiang Z. J. Mater. Chem. 2009, 19, 9068. |
| [103] | Wang X.; Li Z.; Shi J.; Wu H.; Jiang Z.; Zhang W.; Song X.; Ai Q. ACS Catal. 2014, 4, 962. |
| [104] | Aresta M.; Dibenedetto A.; Baran T.; Angelini A.; Labuz P.; Macyk W. Beilstein J. Org. Chem. 2014, 10, 2556. |
| [105] | El-Zahab B.; Donnelly D.; Wang P. Biotechnol. Bioeng. 2008, 99, 508. |
| [106] | Ji X.; Su Z.; Wang P.; Ma G.; Zhang S. ACS Nano 2015, 9, 4600. |
| [107] | Singh R. K.; Singh R.; Sivakumar D.; Kondaveeti S.; Kim T.; Li J.; Sung B. H.; Cho B.-K.; Kim D. R.; Kim S. C.; Kalia V. C.; Zhang Y.-H. P. J.; Zhao H.; Kang Y. C.; Lee J.-K. ACS Catal. 2018, 8, 11085. |
| [108] | Calvin M.; Massini P. Experientia 1952, 8, 445. |
| [109] | Wendell D.; Todd J.; Montemagno C. Nano Lett. 2010, 10, 3231. |
| [110] | Atsumi S.; Higashide W.; Liao J. C. Nat. Biotechnol. 2009, 27, 1177. |
| [111] | Wang W.; Yan Z.-J.; Yuan Y.; Sun F.-X.; Zhao M.; Ren H.; Zhu G.-S. Acta Chim. Sinica 2014, 72, 557 |
| [111] | 王 维; 闫 卓君; 元 野; 孙 福兴; 赵 明; 任 浩; 朱 广山 化学学报 2014, 72, 557 |
| [112] | Jia J.-T.; Wang L.; Zhao Q.; Sun F.-X.; Zhu G.-S. Acta Chim. Sinica 2013, 71, 1492 |
| [112] | 贾 江涛; 王 蕾; 赵 晴; 孙 福兴; 朱 广山 化学学报 2013, 71, 1492 |
/
| 〈 |
|
〉 |