

α-MnO2纳米棒/多孔碳正极材料的制备及水系锌离子电池性能研究
收稿日期: 2020-09-16
网络出版日期: 2020-12-18
基金资助
国家自然科学基金(51863019); 甘肃省自然科学基金(20JR10RA108); 甘肃省高等学校创新基金项目(2020A-013)
Preparation of α-MnO2 Nanorods/Porous Carbon Cathode for Aqueous Zinc-ion Batteries
Received date: 2020-09-16
Online published: 2020-12-18
Supported by
National Natural Science Foundation of China(51863019); Natural Science Foundation of Gansu Province of China(20JR10RA108); Innovation Fund of Gansu Universities(2020A-013)
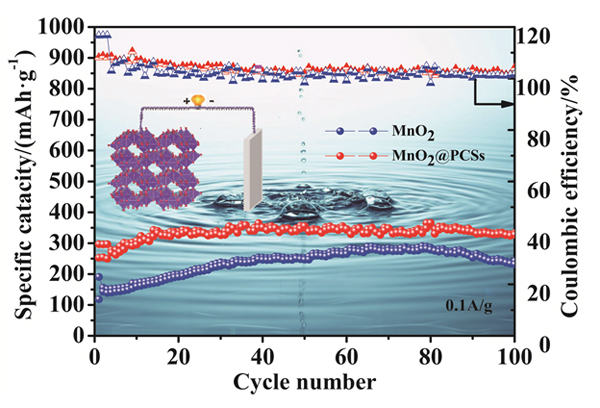
针对水系锌离子电池锰基正极材料存在比容量低、循环稳定性差等问题, 本工作利用水热法制备出棒状结构的α-MnO2, 通过柠檬酸钠高温碳化制备多孔碳, 进而通过超声分散等处理制备出α-MnO2/PCSs复合材料. 三维的多孔网络有助于提高电子导电性, 提供一个稳定的支撑;α-MnO2纳米棒均匀地附着在多孔碳纳米片层表面, 有效地避免α-MnO2的团聚, 从而提高锌离子传输效率. 得益于α-MnO2/PCSs独特的结构优势, 将其作为锌离子电池正极材料, 在电流密度为0.1 A•g–1的条件下循环100次后, 其可逆容量为350 mAh•g–1, 在1 A•g–1的大的电流密度下, 经过1000圈循环后, 容量可达160 mAh•g–1, 展现了优异的循环稳定性能, 有望成为高性能锌离子电池的潜在正极材料.
李燕丽 , 于丹丹 , 林森 , 孙东飞 , 雷自强 . α-MnO2纳米棒/多孔碳正极材料的制备及水系锌离子电池性能研究[J]. 化学学报, 2021 , 79(2) : 200 -207 . DOI: 10.6023/A20090428
Aqueous zinc-ion batteries (ZIBs) have attracted more attention as large-scale energy storage technology due to their high safety, low cost and environmental benignity. To date, numerous cathodes based on manganese dioxide, vanadium dioxide, and polyanionic compounds have been reported. Among them, Manganese oxides have the advantages of low cost, non-toxicity, abundant materials and high working voltage, have been widely explored as promising cathodes for zinc ion batteries. Among them, MnO2 cathodes are particularly desirable candidates for commercialization owing to their tunnel structure and affordability. α-MnO2has (1×1) and (2×2) tunnel structures, and Zn2+ can rapidly inserted and deserted in the tunnel. However, the capacity of manganese dioxide is fast decaying with the cycles due to the poor conductivity of MnO2, which limits its electrochemical performance. Herein, α-MnO2 nanorods are uniformly distributed on the surface of porous carbon nanosheets network (PCSs) by a simple hydrothermal/dispersion method strategy. In the α-MnO2/PCSs architecture, the α-MnO2 nanorods and α-MnO2/PCSs composite were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), high-resolution transmission electron microscopy (HRTEM), etc. The results testified that α-MnO2/PCSs nanorods were firmly adhered on the surface of porous carbon nanosheets, which can effectively avoid the stacking of α-MnO2nanorods, while the high conductive carbon network can improve the electrical conductivity of the composite. The porous network can provide effective electron transfer channels, provide stable hosts for fast Zn2+ extraction/insertion, and prevent the α-MnO2nanorods from stacking each other. Benefiting from the unique porous structure and high conductive network, the α-MnO2/PCSs hybrid shows high reversibility capacity, good rate performance, and outstanding cycling stability. Specifically, α-MnO2/PCSs exhibits high reversible capacity of 350 mAh•g–1 after 100 cycles at 0.1 A•g–1 and maintains a capacity of 160 mAh•g–1 at a high rate of 1 A•g–1 after 1000 cycles, thus making it promising cathode for the high performance ZIBs.

| [1] | Yabuuchi N.; Kubota K.; Dahbi M.; Komaba S. Chem. Rev. 2014, 114, 11636. |
| [2] | Hu X.F.; Sun J.C.; Li Z.F.; Zhao Q.; Chen C.C.; Chen J. Angew. Chem. Int. Ed. 2016, 128, 6592. |
| [3] | Wang L.; Zhao D.-D.; Liu X.; Yu P.; Fu H.G. Acta Chim. Sinica 2017, 75, 231. (in Chinese) |
| [3] | 王蕾, 赵冬冬, 刘旭, 于鹏, 付宏刚, 化学学报, 2017, 75, 231. |
| [4] | Ren T.; Zhuang Q.C.; Hao Y.W.; Cui Y.L. Acta Chim. Sinica 2016, 74, 833. (in Chinese) |
| [4] | 任彤, 庄全超, 郝玉婉, 崔永丽, 化学学报, 2016, 74, 833. |
| [5] | Zhao Q.; Yan Z.H.; Chen C.C.; Chen J. Chem. Rev. 2017, 117, 10121. |
| [6] | Lee J.; Ju J.B.; Cho W.I.; Cho B.W.; Oh S.H. Electrochim. Acta 2013, 112, 138. |
| [7] | Lee B.; Lee H.R.; Kim H.; Chung K.Y.; Cho B.W.; Oh S.H. Chem. Commun. 2015, 51, 9265. |
| [8] | Häupler B.; Rössel C.; Schwenke A.M.; Winsberg J.; Schmidt D.; Wild A.; Schubert U.S. NPG Asia Mater. 2016, 8, e283. |
| [9] | Xu D.W.; Li B.H.; Wei C.G.; He Y.B.; Du H.D.; Chu X.D.; Qin X.Y.; Yang Q.H.; Kang F.Y. Electrochim. Acta 2014, 133, 254. |
| [10] | Li H.F.; Xu C.J.; Han C.P.; Chen Y.Y.; Wei C.G.; Li B.H.; Kang F.Y. J. Electrochem. Soc. 2015, 162, A1439. |
| [11] | Lee B.; Yoon K.; Seo C.R.; Cho B.W.; Lee H.R.; Yoon C.S.; Oh S.H. ChemSusChem 2016, 9, 2948. |
| [12] | Xu C.J.; Du H.D.; Li B.H.; Kang F.Y.; Zeng Y.Q. Electrochem. Solid-State Lett. 2009, 12, A61. |
| [13] | Pan H.L.; Shao Y.Y.; Yan P.F.; Cheng Y.W.; Han K.S.; Nie Z.M.; Wang C.M.; Yang J.H.; Li X.L.; Bhattacharya P.; Mueller K.T.; Liu J. Nat. Energy 2016, 1, 16039. |
| [14] | Wang J.; Wang J.G.; Liu H.; Wei C.; Kang F. J. Mater. Chem. 2019, 7, 13727. |
| [15] | Alfaruqi M.H.; Mathew V.; Song J.J.; Kim S.; Islam S.; Pham D.T.; Jo J.; Kim S.; Baboo J.P.; Xiu Z.L.; Lee K.S.; Sun Y.K.; Kim J. Chem. Mater. 2017, 29, 1684. |
| [16] | He P.; Yan M.Y.; Zhang G.B.; Sun R.M.; Chen L.N.; An Q.Y.; Mai L.Q. Adv. Energy Mater. 2017, 7, 1601920. |
| [17] | Zeng Y.X.; Zhang X.Y.; Meng Y.; Yu M.H.; Yi J.N.; Wu Y.Q.; Lu X.H.; Tong Y.X. Adv. Mater. 2017, 29, 1700274. |
| [18] | Qiu W.D.; Li Y.; You A.; Zhang Z.M.; Li F.G.; Lu X.H.; Tong Y.X. J. Mater. Chem. 2017, 5, 28. |
| [19] | Alfaruqi M.H.; Gim J.; Kim S.J.; Song J.J.; Jo J.; Kim S.; Mathew V.; Kim J. J. Power Sources 2015, 288, 320. |
| [20] | Alfaruqi M.H.; Mathew V.; Gim J.; Kim S.; Song J.J.; Baboo J.P.; Choi S.H.; Kim J. Chem. Mater. 2015, 27, 3609. |
| [21] | Alfaruqi M.H.; Gim J.; Kim S.; Song J.J.; Pham D.T.; Jo J.; Xiu Z.L.; Mathew V.; Kim J. Electrochem. Commun. 2015, 60, 121. |
| [22] | Fu Y.Q.; Wei Q.L.; Zhang G.X.; Wang X.M.; Zhang J.H.; Hu Y.F.; Wang D.N.; Zuin L.; Zhou T.; Wu Y.C.; Sun S.H. Adv. Energy Mater. 2018, 8, 1801445. |
| [23] | Deng Z.H.; Huang J.D.; Liu J.; Ren L.; Zhu L.Z.; Xiao X.Y.; Tan M.X. Mater. Lett. 2019, 248, 207. |
| [24] | Yan T.T.; Xing G.L.; Ben T. Acta Chim. Sinica 2018, 76, 366. (in Chinese) |
| [24] | 闫婷婷, 邢国龙, 贲腾, 化学学报, 2018, 76, 366. |
| [25] | Tong Z.K.; Fang S.; Zheng H.; Zhang X.G. Acta Chim. Sinica 2016, 74, 185. (in Chinese) |
| [25] | 童震坤, 方姗, 郑浩, 张校刚, 化学学报, 2016, 74, 185. |
| [26] | Yang B.B.; Wang J.; Bin D.; Zhu M.S.; Yang P.; Du Y.K. J. Mater. Chem. 2015, B3, 7440. |
| [27] | Huang Y.; Liu H.; Gong L.; Hou Y.L.; Li Q. J. Power Sources 2017, 347, 29. |
| [28] | Wang D.H.; Li H.F.; Liu Z.X.; Tang Z.J.; Liang G.J.; Mo F.N.; Yang Q.; Ma L.T.; Zhi C.Y. Small 2018, 14, 1803978. |
| [29] | Wang Q.F.; Zou R.Q.; Xia W.; Ma J.; Qiu B.; Mahmood A.; Zhao R.; Yang Y.Y.C.; Xia D.G.; Xu Q. Small 2015, 11, 2511. |
| [30] | Guo S.P.; Li J.C.; Ma Z.; Chi Y.; Xue H.G. J. Mater. Sci. 2016, 52, 2345. |
| [31] | Toupin M.; Brousse T.; Daniel B. Chem. Mater. 2004, 16, 3184. |
| [32] | Wang J.J.; Wang J.G.; Liu H.Y.; Wei C.G.; Kang F.Y. J. Mater. Chem. A 2019, 7, 13727. |
| [33] | Fang G.Z.; Zhou J.; Pan A.Q.; Liang S.Q. ACS Energy Lett. 2018, 3, 2480. |
| [34] | Zhou J.; Shan L.T.; Tang B.Y.; Liang S.Q. Chin. Sci. Bull. 2020, 65, 3562. (in Chinese) |
| [34] | 周江, 单路通, 唐博雅, 梁叔全, 科学通报, 2020, 65, 3562. |
| [35] | Huang J.T.; Zhou J.; Liang S.Q. Acta Phys.-Chim. Sinica 2021, 37, 2005020. (in Chinese) |
| [35] | 黄江涛, 周江, 梁叔全, 物理化学学报, 2021, 37, 2005020. |
| [36] | Huang J.H.; Wang Z.; Hou M.; Dong X.L.; Liu Y.; Wang Y.; Xia Y. Nat. Commun. 2018, 9, 2906. |
| [37] | Xu C.J.; Li B.H.; Du H.D.; Kang F.Y. Angew. Chem. Int. Ed. 2012, 51, 933. |
| [38] | Zuo S.Y.; Xu X.J.; Ji S.M.; Wang Z.S.; Liu Z.B.; Liu J. Chem. Eur. J. 2020, 26, 1. |
| [39] | Wang S.Y.; Wei Q.L.; He P.; Chen Y.; Xu X.; An Q.Y.; Shuang Y.; Shao Y.Y.; Mai L.Q.; Liu J.; Yang J.H. Adv. Mater. 2018, 30, 1703725. |
| [40] | Zhang Y.; Deng S.J.; Luo M.; Pan G.X.; Zeng Y.X.; Lu X.H.; Ai C.Z.; Liu Q.; Xiong Q.Q.; Wang X.L.; Xia X.H.; Tu J.P. Small 2019, 15, 1905452. |
| [41] | Fen X.M.; Huang X.W.; Tan Z.; Zhao B.; Tan S.T. Acta Chim. Sinica 2011, 69, 653. (in Chinese) |
| [41] | 冯小明, 黄先威, 谭卓, 赵斌, 谭松庭, 化学学报, 2011, 69, 653. |
| [42] | Li C.P.; Xie X.S.; Liang S.Q.; Zhou J. Energy Environ. Mater. 2020, 3, 146. |
| [43] | Liu N.N.; Wu X.; Yin Y.Y.; Chen A.S.; Zhao C.Y.; Guo Z.K.; Fan L.S.; Zhang N.Q. ACS Appl. Mater. Interfaces 2020, 12, 28199. |
/
| 〈 |
|
〉 |