

铜催化不对称去对称化分子内烯基C—N偶联反应
收稿日期: 2021-01-12
网络出版日期: 2021-02-22
基金资助
项目受国家自然科学基金(21772066); 广东省重点研发领域项目(2020B010188001)
Copper(I)-Catalyzed Asymmetric Desymmetric Intramolecular Alkenyl C—N Coupling Reaction
Received date: 2021-01-12
Online published: 2021-02-22
Supported by
National Natural Science Foundation of China(21772066); Key-Area Research and Development Program of Guangdong Province(2020B010188001)
邓卓基 , 欧阳溢凡 , 敖运林 , 蔡倩 . 铜催化不对称去对称化分子内烯基C—N偶联反应[J]. 化学学报, 2021 , 79(5) : 649 -652 . DOI: 10.6023/A21010006
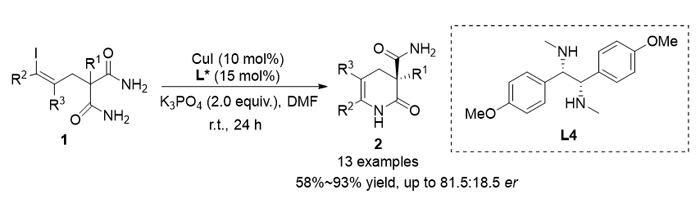
Enantioselective desymmetrization is a powerful strategy in asymmetric synthesis. By differentiating two identical enantiotopic functional groups through simple transformations, asymmetric desymmetrizations provide efficient protocols for the synthesis of chiral compounds from easily available starting materials. The strategy has been successfully applied in a broad range of organocatalytic and transition metal-catalyzed asymmetric reactions. Copper-catalyzed coupling reactions are one of the most important methods for the construction of aryl or alkenyl carbon-heteroatom bonds. But the asymmetric coupling reactions remain a great challenge. It may be because that the bonds are generally formed between sp2-hybrized carbon and heteroatoms like N or O, and no chiral carbon centers were involved in the bond formation process. By utilizing desymmetrization strategies, we have developed a variety of copper-catalyzed enantioselective aryl carbon-heteroatom bond coupling reactions. However, the research on copper-catalyzed asymmetric alkenyl C-heteroatom coupling is rarely reported, and only one example of enantioselective copper-catalyzed alkenyl C—O bond coupling was achieved recently by Liu and co-workers via the desymmetrization strategy. In a previous work, we reported a desymmetric intramolecular aryl C—N coupling reaction of 2-(2-iodobenzyl)malonamides for the synthesis of chiral 2-oxo-1,2,3,4-tetrahydroquinoline-3-carboxamides. During the course, we believed that such a desymmetrization strategy should also be applicable to alkenyl C—N bond coupling reactions. To explore this idea, in this work, an enantioselective alkenyl C—N coupling is developed. It is a copper- catalyzed intramolecular desymmetric reaction with 2-(3-iodoallyl)malonamides as the substrates. Under the catalysis of 10 mol% CuI and 15 mol% of chiral diamine ligand, the reactions of 2-(3-iodoallyl)malonamides proceeded smoothly at room temperature inN,N-dimethylformamide (DMF), with K3PO4 as the base. It afforded the desired 2-oxo-1,2,3,4-tetrahydro- pyridine-3-carboxamide products bearing quaternary stereogenic carbon centers in high yields and moderate enantioselectivities. An example of double alkenyl C—N coupling for the synthesis of chiral 2,8-diazaspiro[5.5]undeca-3,9-diene-1,7-dione spirocyclic product was also demonstrated. Although the enantioselectivity is unsatisfactory, the reactions expanded the scope of copper-catalyzed asymmetric C—N coupling from aryl to alkenyl C—N coupling. It may find further applications in the synthesis of chiral heterocycles.
| [1] | Rovis, T. In New Frontiers in Asymmetric Catalysis, Eds.: Mikami, K.; Lautens, M., John Wiley & Sons, Inc., New York, 2007, pp.275~309. |
| [2] | For selected reviews, see: (a) Atodiresei, I.; Schiffers, I.; Bolm, C. Chem. Rev. 2007,107,5683. |
| [2] | (b) Díaz-de-Villegas, M. D.; Gálvaz, J. A.; Etayo, P.; Badorrey, R.; López-Ram-de-Víu, M. P. Chem.-Eur. J. 2012, 18,13920. |
| [2] | (c) Zeng, X.-P.; Cao, Z.-Y.; Wang, Y.-H.; Zhou, F.; Zhou, J. Chem. Rev. 2016, 116,7330. |
| [2] | (d) Zhu, R.-Y.; Liao, K.; Yu, J.-S.; Zhou, J. Acta Chim. Sinica 2020, 78,193. (in Chinese). |
| [2] | ( 朱仁义, 廖奎, 余金生, 周剑, 化学学报, 2020, 78,193.) |
| [3] | For selected examples about asymmetric desymmetrization,see: (a) Sato, Y.; Sodeoka, M.; Shibasaki, M. J. Org. Chem. 1989,54,4738. |
| [3] | (b) Willis, M. C.; Powell, L. H.; Claverie, C. K.; Watson, S. J. Angew. Chem. Int. Ed. 2004, 43,1249. |
| [3] | (c) Albicker, M. R.; Cramer, N. Angew. Chem. Int. Ed. 2009, 48,9139. |
| [3] | (d) Wasa, M.; Engle, K. M.; Lin, D. W.; Yoo, E. J.; Yu, J.-Q. J. Am. Chem. Soc. 2011, 133,19598. |
| [3] | (e) Sattely, E. S.; Meek, S. J.; Malcolmson, S. J.; Schrock, R. R.; Hoveyda, A. H. J. Am. Chem. Soc. 2009, 131,943. |
| [3] | (f) Zhou, F.; Tan, C.; Tang, J.; Zhang, Y.-Y.; Gao, W.-M.; Wu, H.-H.; Yu, Y.-H.; Zhou, J. J. Am. Chem. Soc. 2013, 135,10994. |
| [3] | (g) Arai, M. A.; Kuraishi, M.; Arai, T.; Sasai, H. J. Am. Chem. Soc. 2001, 123,2907. |
| [3] | (h) Bocknack, B. M.; Wang, L.-C.; Krische, M. J. Proc. Natl. Acad. Sci. U. S. A. 2004, 101,5421. |
| [3] | (i) Linclau, B.; Cini, E.; Oakes, C. S.; Josse, S.; Light, M.; Ironmonger, V. Angew. Chem. Int. Ed. 2011, 51,1232. |
| [3] | (j) Aikawa, K.; Okamoto, T.; Mikami, K. J. Am. Chem. Soc. 2012, 134,10329. |
| [3] | (k) Li, B.-L.; Gao, W.-Y.; Zhang, S.-Q; Han, X.-Q.; Lu, J.; Liang, R.-X.; Hong, X.; Jia, Y.-X. Chin. J. Chem. 2019, 37,63. |
| [3] | (l) Zhou, F.; Zhou, J. Chin. J. Org. Chem. 2020, 40,2180. (in Chinese). |
| [3] | ( 周锋, 周剑, 有机化学, 2020, 40,2180.) |
| [4] | For selected reviews: (a) Cai, Q.; Zhou, W. Chin. J. Chem. 2020,38,879. |
| [4] | (b) Dai, L. Prog. Chem. 2018, 30,1257. |
| [4] | (c) Bhunia, S.; Pawar, G. G.; Kumar, S. V.; Jiang, Y.; Ma, D. Angew. Chem. Int. Ed. 2017, 56,16136. |
| [4] | (d) Sambiagio, C.; Marsden, S. P.; Blacker, M. A.; McGowan, P. C. Chem. Soc. Rev. 2014, 43,3525. |
| [4] | (e) Rao, H.; Fu, H. Synlett 2011,745. |
| [4] | (f) Ma, D.; Cai, Q. Acc. Chem. Res. 2008, 41,1450. |
| [4] | (g) Beletskaya, I.; Cheprakov, A. V. Coord. Chem. Rev. 2004, 248,2337. |
| [4] | (h) Ley, S. V. Angew. Chem. Int. Ed. 2003, 42,5400. |
| [5] | For selected reviews about applications of copper-catalyzed coupling reactions,see: (a) Maaliki, C.; Thiery, E.; Thibonnet, J. Eur. J. Org. Chem. 2017,2,209. |
| [5] | (b) Okano, K.; Tokuyama, H.; Fukuyama, T. Chem. Commun. 2014, 50,13650. |
| [5] | (c) Lin, H.; Sun, D. Org. Prep. Proceed. Int. 2013, 45,341. |
| [5] | (d) Cacchi, S.; Fabrizi, G.; Goggiamani, A. Org. Biomol. Chem. 2011, 9,641. |
| [5] | (e) Evano, G.; Toumi, M.; Coste, A. Chem. Commun. 2009,4166. |
| [5] | (f) Evano, G.; Blanchard, N.; Toumi, M. Chem. Rev. 2008, 108,3054. |
| [6] | Zhou, F.; Cai, Q. Beilstein J. Org. Chem. 2015, 11,2600. |
| [7] | For selected asymmetric examples of cyclic diaryliodonius with nucleophiles, see: (a) Zhao, K.; Duan, L.; Xu, S. Jiang, J. Fu, Y. Gu, Z. Chem 2018,4,599. |
| [7] | (b) Guo, W.; Gu, J.; Gu, Z. Org. Lett. 2020, 22,7622. |
| [7] | (c) Chao, Z.; Ma, M.; Gu, Z. Org. Lett. 2020, 22,6441. |
| [7] | (d) Li, Q.; Zhang, M.; Zhan, S.; Gu, Z. Org. Lett. 2019, 21,6374. |
| [7] | (e) Hou, M.; Deng, R.; Gu, Z. Org. Lett. 2018, 20,5799. |
| [7] | (f) Li, B.; Chao, Z.; Li, C.; Gu, Z. J. Am. Chem. Soc. 2018, 140,9400. |
| [8] | Zhou, F.; Liu, J.; Cai, Q. Synlett 2016, 27,664. |
| [9] | He, N.; Huo, Y.; Liu, J.; Huang, Y.; Zhang, S.; Cai, Q. Org. Lett. 2015, 17,374. |
| [10] | Liu, J.; Tian, Y.; Shi, J.; Zhang, S.; Cai, Q. Angew. Chem. Int. Ed. 2015, 54,10917. |
| [11] | For a copper-catalyzed asymmetric alkenyl C—O coupling,see: Cai, J.; Wang, Z.-K.; Usman, M.; Lu, Z.-W.; Hu, X.-D.; Liu, W.-B. Org. Lett. 2019, 21,8852. |
| [12] | For diamine ligands in copper-catalyzed coupling reactions,see: Surry, D. S.; Buchwald, S. L. Chem. Sci. 2010, 1,13. |
| [13] | For examples of chiral diamine ligands in copper-catalyzed asymmetric coupling reactions,see: (a) Liu, Y.; Wang, Z.; Guo, B.; Cai, Q. Tetrahedron Lett. 2016,57,2379. |
| [13] | (b) Yang, W.; Liu, Y.; Zhang, S.; Cai, Q. Angew. Chem. Int. Ed. 2015, 54,8805. |
| [13] | (c) Zhang, Y.; Wang, Q.; Wang, T.; He, H.; Yang, W.; Zhang, X.; Cai, Q. J. Org. Chem. 2017, 82,1458. |
/
| 〈 |
|
〉 |