

液相合成彩色氮化碳及其光电化学特性研究
收稿日期: 2020-12-19
网络出版日期: 2021-03-12
基金资助
重庆市留学人员创新支持计划重点项目(CX2017023); “成渝地区双城经济圈建设”科技创新项目(KJCX2020028); 国家自然科学基金青年基金(12004061); 重庆市教育委员会科学技术研究重点项目(KJZD-K201800602); 重庆自然科学基金面上项目(cstc2019jcyj-msxmX0525)
Study on Photoelectrochemical Properties of Colorful Carbon Nitrides Synthesized in Liquid-Phase
Received date: 2020-12-19
Online published: 2021-03-12
Supported by
Venture & Innovation Support Program for Chongqing Oversea Returnee(CX2017023); Construction of Double City Economic Circle in Chengdu Chongqing Region Scientific and Technological Innovation Project(KJCX2020028); National Natural Science Foundation of China(12004061); Science and Technology Research Program of Chongqing Municipal Education(KJZD-K201800602); Chongqing Natural Science Foundation(cstc2019jcyj-msxmX0525)
何利蓉 , 唐笑 , 张灵 , 李艳虹 , 相国涛 , 周贤菊 , 凌发令 , 姚璐 , 蒋浩 . 液相合成彩色氮化碳及其光电化学特性研究[J]. 化学学报, 2021 , 79(4) : 506 -512 . DOI: 10.6023/A20120575
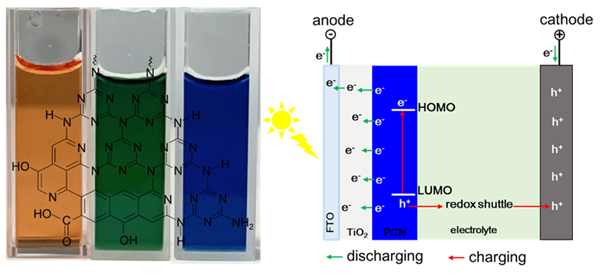
Reducing the band gap value and obtaining an ordered two-dimensional microstructure are crucial to improving the photoelectrochemical performance of carbon nitrides. By adjusting the ratio of urea and citric acid and ripening the precursor at the room temperature, we synthesize the carbon nitride materials with different colors of orange, green and blue. The resultant blue sample has a narrowed band gap of 1.74 eV and the strong absorption to the light in 550~700 nm wavelength range. The photoelectrochemical solar cell is fabricated using the synthesized carbon nitrides as light absorber, TiO2 as electron transporter and I3–/I– as the redox shuttle in electrolyte. The orange carbon nitride has typical photoelectric conversion performance with Jsc=0.65 mA•cm–2, Voc=530 mV and ff=0.64. The blue carbon nitride has obvious enhanced Jsc of 1.03 mA•cm–2 owing to the obvious enhanced visible light absorption, while the deeply decreased ff of 0.33. The cyclic voltammetry tests demonstrate the electrode reaction properties of the fabricated carbon nitride-based cell under illumination. It turns out that the cell based on the blue carbon nitride has obvious photo-induce pseudocapacitive behavior with an area specific capacitance of 4.9 mF•cm–2. TEM (Transmission electron microscope), XRD (X-ray diffraction), FTIR (Fourier transform infrared spectroscopy) and XPS (X-ray photoelectron spectroscopy) reveal the microstructure characteristics of the colored carbon nitrides. The orange one is carbon nitride quantum dots with the particle size less than 10 nm. The blue one is a carbon nitride polymer containing graphite domains with oxidized boundaries, forming three-dimensional porous structure constructed by an ordered two-dimensional network. The green one has a mixture microstructure as well as the medium photoelectrochemical properties between the former two, which are both photoelectric conversion and photo-induced charge storage performance.
| [1] | Ma, C.; Wu, J.; Zhu, L.; Han, X.; Ruan, W.; Song, W.; Wang, X.; Zhao, B. Acta Chim. Sinica 2019, 77,1024. (in Chinese) |
| [1] | ( 马超, 武佳炜, 朱琳, 韩晓霞, 阮伟东, 宋薇, 王旭, 赵冰, 化学学报, 2019, 77,1024.) |
| [2] | Su, S.-J.; Lai, Q.-X.; Liang, Y.-Y. Acta Chim. Sinica 2015, 73,735. (in Chinese) |
| [2] | ( 苏善金, 来庆学, 梁彦瑜, 化学学报, 2015, 73,735.) |
| [3] | Yang, H.; Wang, Z.; Liu, S.-Q.; Shen, Y.-F.; Zhang, Y.-J. Chin. Chem. Lett. 2020, 31,3047. |
| [4] | Hou, Y.; Wen, Z.; Cui, S.; Guo, X.; Chen, J. Adv. Mater. 2013, 25,6291. |
| [5] | Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. Nat. Mater. 2009, 8,76. |
| [6] | Yang, X.-H.; Wang, H.-J.; Lu, X.-F.; Cui, D.-L.; Zhang, S.-Y. Acta Chim. Sinica 2009, 67,1166. (in Chinese) |
| [6] | ( 杨晓晖, 王红军, 陆希峰, 崔得良, 张树永, 化学学报, 2009, 67,1166.) |
| [7] | Li, F.; Tang, M.; Li, T.; Zhang, L.; Hu, C. Appl. Catal., B: Environ. 2020, 268,118397. |
| [8] | Hao, X.-Q.; Yang, H.; Jin, Z.-L.; Xu, J.; Min, S.-X.; Lü, G.-X. Acta Phys.-Chim. Sin. 2016, 32,2581. (in Chinese) |
| [8] | ( 郝旭强, 杨浩, 靳治良, 续京, 敏世雄, 吕功煊, 物理化学学报, 2016, 32,2581.) |
| [9] | Su, P.; Guo, H.-L.; Peng, S.; Ning, S.-K. Acta Phys.-Chim. Sin. 2012, 28,2745. (in Chinese) |
| [9] | ( 苏鹏, 郭慧林, 彭三, 宁生科, 物理化学学报, 2012, 28,2745.) |
| [10] | Safaei, J.; Mohamed, N.A.; Noh, M.F. M.; Soh, M.F.; Ludin, N.A.; Ibrahim, M.A.; Roslam, W.N.; Isahakb, W.; Teridi, M.A. M. J. Mater. Chem. A 2018, 6,22346. |
| [11] | Li, Z.; Wu, S.; Zhang, J.; Yuan, Y.; Wang, Z.; Zhu, Z. Solar RRL 2019, 4,1900413. |
| [12] | Liu, J.; Zhang, Y.; Zhang, L.; Xie, F.; Vasileff, A.; Qiao, S. Adv. Mater. 2019, 31,1901261. |
| [13] | Kong, D.; Gao, Y.; Xiao, Z.; Xu, X.; Li, X.; Zhi, L. Adv. Mater. 2018, 31,1804973. |
| [14] | Inagaki, M.; Tsumura, T.; Kinumoto, T.; Toyoda, M. Carbon 2019, 141,580. |
| [15] | Zhao, T.-T.; Zhou, Q.; Lv, Y.-Q.; Han, D.; Wu, K.-Q.; Zhao, L.-F.; Shen, Y.-F.; Liu, S.-Q.; Zhang, Y.-J. Angew. Chem. Int. Ed. 2019, 59,1139. |
| [16] | Qi, S.; Ma, X.; Yang, B.; Sun, L.; Li, W.; Zhao, M. Carbon 2019, 149,234. |
| [17] | Algara-Siller, G.; Severin, N.; Chong, S.Y. Angew. Chem. Int. Ed. 2014, 53,7450. |
| [18] | Kumar, P.; Vahidzadeh, E.; Thakur, U.K.; Kar, P.; Alam, K.M.; Goswami, A.; Mahdi, N.; Cui, K.; Bernard, G.M.; Michaelis, V.K.; Shankar, K. J. Am. Chem. Soc. 2019, 141,5415. |
| [19] | Lin, L.; Yu, Z.; Wang, X. Angew. Chem. Int. Ed. 2019, 58,6164. |
| [20] | Yang, Q.; Yu, S.; Fu, P.; Yu, W.; Liu, Y.; Liu, X.; Feng, Z.; Guo, X.; Li, C. Adv. Funct. Mater. 2020, 30,1910205. |
| [21] | Mo, Z.; Xu, H.; Chen, Z.; She, X.; Song, Y.; Lian, J.; Zhu, X.; Yan, P.; Lei, Y.; Yuan, S.; Li, H. Appl. Catal., B 2019, 241,452. |
| [22] | Zheng, F.; Yang, Y.; Chen, Q. Nature Commun. 2014, 5,1. |
| [23] | Si, Y.; Samulski, E.T. Nano Lett. 2008, 8,1679. |
| [24] | Podjaski, F.; Kröger, J.; Lotsch, B.V. Adv. Mater. 2018, 30,1705477. |
| [25] | Geng, D.; Yang, S.; Zhang, Y.; Yang, J.; Liu, J.; Li, R.; Sham, T.; Sun, X.; Ye, S.; Knights, S. Appl. Surf. Sci. 2011, 257,9193. |
| [26] | Hu, C.; Liu, Y.; Yang, Y.; Cui, J.; Huang, Z.; Wang, Y.; Yang, L.; Wang, H.; Xiao, Y.; Rong, J. J. Mater. Chem. B 2013, 1,39. |
| [27] | Usachov, D.; Vilkov, O.; Grüneis, A.; Haberer, D.; Fedorov, A.; Adamchuk, V.K.; Preobrajenski, A.B.; Dudin, P.; Barinov, A.; Oehzelt, M.; Laubschat, C.; Vyalikh, D.V. Nano Lett. 2011, 11,5401. |
| [28] | Xu, J.; Xu, F.; Qian, M.; Xu, F.; Hong, Z.; Huang, F. Adv. Mater. 2017, 29,1701674. |
| [29] | Hou, S.; Cai, X.; Wu, H.; Yu, X.; Peng, M.; Yan, K.; Zou, D. Energy Environ. Sci. 2013, 6,3356. |
| [30] | Papailias, I.; Todorova, N.; Giannakopoulou, T.; Ioannidisa, N.; Dallasa, P.; Dimotikalib, D.; Trapalis, C. Appl. Catal. B: Environ. 2020, 268,118733. |
| [31] | Hu, X.; Zeng, X.; Liu, Y.; Lu, J.; Yuan, S.; Yin, Y.; Hu, J.; McCarthy, D.T.; Zhang, X. Appl. Catal. B: Environ. 2020, 268,118466. |
| [32] | Ong, W.-J.; Tan, L.-L.; Chai, S.-P. Nano Energy 2015, 13,757. |
/
| 〈 |
|
〉 |