

细菌纤维素基柔性锌离子电池正极的构筑及性能研究
收稿日期: 2021-01-31
网络出版日期: 2021-03-30
基金资助
项目受国家重点研发计划(2018YFE0201704); 国家自然科学基金(21771059); 国家自然科学基金(21631004); 黑龙江省自然科学基金(YQ2019B007)
Study on the Construction and Properties of Bacterial Cellulose-Based Cathode for Flexible Zn-Ion Batteries
Received date: 2021-01-31
Online published: 2021-03-30
Supported by
National Key R&D Program of China(2018YFE0201704); National Natural Science Foundation of China(21771059); National Natural Science Foundation of China(21631004); Natural Science Foundation of Heilongjiang Province(YQ2019B007)
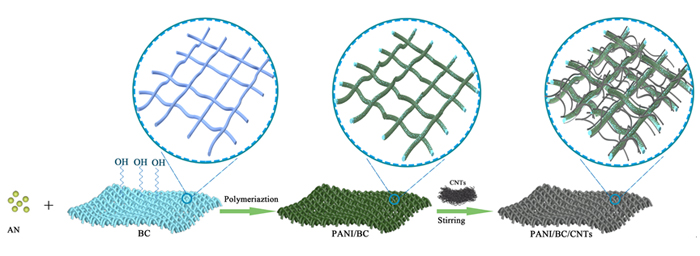
柔性锌离子电池(ZIBs)具有高安全性、低成本和高能量密度等优势, 但是现有的ZIBs柔性电极难以兼具高电化学性能和力学稳定性. 其中, 缺少适合的基底材料是限制柔性电极发展的关键. 本工作中, 以细菌纤维素(BC)为基底材料, 结合原位聚合及真空过滤方法, 制备了具有3D多孔结构的BC/聚苯胺/碳纳米管(BC/PANI/CNTs)柔性电极. BC固有的高抗拉伸强度和超细纳米纤维网络结构等特点, 在赋予柔性电极高弯曲特性的同时, 还有利于活性物质的负载及电解液离子的快速扩散. 结果表明, BC/PANI/CNTs具有高柔韧性、7.3 mg/cm2的负载量和157 mAh/g的比容量. 以BC/PANI/CNTs电极构建的准固态ZIBs展现了109 mAh/g的比容量, 且200次充放电循环后容量保持率大于90%.
张欣欣 , 刘荣 , 王蕾 , 付宏刚 . 细菌纤维素基柔性锌离子电池正极的构筑及性能研究[J]. 化学学报, 2021 , 79(5) : 670 -677 . DOI: 10.6023/A21010037
Flexible Zn-ion batteries (ZIBs) have been considered as desirable candidate of flexible energy storages due to its high safety, low cost and high energy density. However, there is a challenge that ZIBs can possess both high electrochemistry properties and good mechanical stability. Among them, the lack of suitable substrate material has long been the major obstacles against the development of flexible electrode for ZIBs. In this work, we prepared the bacterial cellulose/polyaniline/ carbon nanotubes (BC/PANI/CNTs) flexible electrode through a simple in-situpolymerization with subsequent vacuum filtration. Typically, the BC suspension and aniline monomer were dissolved into HCl solution. After the ammonium persulfate dispersed in another HCl aqueous solution, the obtained solution was subsequently added dropwise into the above mixture in an ice bath. Then, the acid treated CNTs was added to achieve the whole dispersion. The resulted film was collected by vacuum filtration and the BC/PANI/CNTs flexible electrode was finally obtained by further dried in an oven. Benefiting from the BC substrate, the BC/PANI/CNTs flexible electrode possesses a 3D porous structure, which can provide the rapid diffusion channel of electrolyte ion and improve the contact between the active material and electrolyte ion. Moreover, the BC substrate endow the BC/PANI/CNTs electrode with high flexibility and large mass loading of active materials. The scanning electron microscopy (SEM) image of the BC/PANI/CNTs electrode shows that the PANI is uniformly anchored on the surface of BC nanofiber, and the CNTs is dispersed in the porous network and connected with BC/PANI nanofibers. Furthermore, the X-ray diffraction (XRD), Raman spectra, Fourier transform infrared spectra (FT-IR) and X-ray photoelectron spectroscopy (XPS) confirm the structure of the BC/PANI/CNTs. The BC/PANI/CNTs, tested as a flexible cathode for ZIBs, achieve both good electrochemical performance, such as mass loading of 7.3 mg/cm2, gravimetric capacity of 157 mAh/g, areal capacity of 1.148 mAh/cm2, and excellent mechanical flexibility, which can be bended, twisted and rolled. To further explore the application value of this flexible electrode in electronic equipment, the quasi-solid-state ZIBs was prepared with the BC/PANI/CNTs as cathode, the Zn/carbon cloth (CC) as anode, and the ZnSO4/polyvinyl alcohol (PVA) as gel electrolyte, respectively. The assembled quasi-solid-state ZIBs deliver a specific capacity of 109 mAh/g, and over 90% of the initial capacity is retained after 200 charge/discharge cycles at a low current density of 0.5 mA/cm2. The low-cost BC/PANI/CNTs flexible electrode has excellent performance and mechanical property, providing a feasible scheme for the scalable application of ZIBs.

Key words: Zn-ion battery; flexible electrode; bacterial cellulose; polyaniline; CNTs
| [1] | Lei, H.; Wang, Z. L.; Yang, F.; Huang, X. Q.; Liu, J. H.; Liang, Y. Y.; Xie, J. P.; Javed, M. S.; Lu, X. H.; Tan, S. Z.; Mai, W. J. Nano Energy 2020, 68,104293. |
| [2] | Li, H. F.; Han, C. P.; Huang, Y.; Huang, Y.; Zhu, M. S.; Pei, Z. X.; Xue, Q.; Wang, Z. F.; Liu, Z. X.; Tang, Z. J.; Wang, Y. K.; Kang, F. Y.; Li, B. H.; Zhi, C. Y. Energy Environ. Sci. 2018, 11,941. |
| [3] | Liao, M.; Wang, J.; Ye, L.; Sun, H.; Wen, Y.; Wang, C.; Sun, X.; Wang, B.; Peng, H. Angew. Chem., Int. Ed. 2020, 59,2273. |
| [4] | Liang, H.; Cao, Z.; Ming, F.; Zhang, W.; Anjum, D. H.; Cui, Y.; Cavallo, L.; Alshareef, H. N. Nano Lett. 2019, 19,3199. |
| [5] | Li, Y.-L.; Yu, D.-D.; Lin, S.; Sun, D.-F.; Lei, Z.-Q. Acta Chim. Sinica 2021, 79,200. (in Chinese). |
| [5] | ( 李燕丽, 于丹丹, 林森, 孙东飞, 雷自强, 化学学报, 2021, 79,200.) |
| [6] | Wu, F.; Gao, X.; Xu, X.; Jiang, Y.; Gao, X.; Yin, R.; Shi, W.; Liu, W.; Lu, G.; Cao, X. ChemSusChem 2020, 13,1537. |
| [7] | Ma, Y.; Xie, X.; Lv, R.; Na, B.; Ouyang, J.; Liu, H. ACS Sustainable Chem. Eng. 2018, 6,8697. |
| [8] | Liu, J.-T.; Xie, Y.; Gao, Q.; Cao, F.-H.; Qin, L.; Wu, Z.-Y.; Zhang, W.; Li, H.; Zhang, C.-L. Eur. J. Inorg. Chem. 2020, 2020,581. |
| [9] | Cong, H.-P.; Ren, X.-C.; Wang, P.; Yu, S.-H. Energy Environ. Sci. 2013, 6,1185. |
| [10] | Yano, H.; Sugiyama, J.; Nakagaito, A. N.; Nogi, M.; Matsuura, T.; Hikita, M.; Handa, K. Adv. Mater. 2005, 17,153. |
| [11] | Hu, W.; Chen, S.; Xu, Q.; Wang, H. Carbohydr. Polym. 2011, 83,1575. |
| [12] | Zhang, X.; He, M.; He, P.; Li, C.; Liu, H.; Zhang, X.; Ma, Y. Appl. Surf. Sci. 2018, 433,419. |
| [13] | Li, S.; Huang, D.; Zhang, B.; Xu, X.; Wang, M.; Yang, G.; Shen, Y. Adv. Energy Mater. 2014, 4,1301655. |
| [14] | Wang, Y.; Wang, X.; Li, X.; Bai, Y.; Xiao, H.; Liu, Y.; Liu, R.; Yuan, G. Adv. Funct. Mater. 2019, 29,1900326. |
| [15] | Liu, R.; Ma, L.; Niu, G.; Li, X.; Li, E.; Bai, Y.; Yuan, G. Adv. Funct. Mater. 2017, 27,1701635. |
| [16] | Ma, L.; Liu, R.; Niu, H.; Xing, L.; Liu, L.; Huang, Y. ACS Appl. Mater. Interfaces 2016, 8,33608. |
| [17] | Wang, Y.; Wang, X.; Li, X.; Bai, Y.; Xiao, H.; Liu, Y.; Liu, R.; Yuan, G. Adv. Funct. Mater. 2019, 29,1900326. |
| [18] | Kim, C.; Ahn, B. Y.; Wei, T. S.; Jo, Y.; Jeong, S.; Choi, Y.; Kim, I. D.; Lewis, J. A. ACS Nano 2018, 12,11838. |
| [19] | Cao, H.; Wan, F.; Zhang, L.; Dai, X.; Huang, S.; Liu, L.; Niu, Z. J. Mater. Chem. A 2019, 7,11734. |
| [20] | Li, X.; Lv, R.; Zou, S.; Na, B.; Liu, P.; Ma, Y.; Liu, H. Compos. Sci. Technol. 2019, 180,71. |
| [21] | Zhang, Y.; Wang, Q.; Bi, S.; Yao, M.; Wan, F.; Niu, Z. Nanoscale 2019, 11,17630. |
| [22] | Shi, H. Y.; Ye, Y. J.; Liu, K.; Song, Y.; Sun, X. Angew. Chem., Int. Ed. 2018, 57,16359. |
| [23] | Wan, F.; Zhang, L.; Wang, X.; Bi, S.; Niu, Z.; Chen, J. Adv. Funct. Mater. 2018, 28,1804975. |
| [24] | Han, J.; Wang, K.; Liu, W.; Li, C.; Sun, X.; Zhang, X.; An, Y.; Yi, S.; Ma, Y. Nanoscale 2018, 10,13083. |
| [25] | Gao, Z.-Z.; Tong, H.; Chen, J.-H.; Yue, S.-H.; Bai, W.-L.; Zhang, X.-G.; Pan, Y.-F.; Shi, M.; Song, Y.-X. Acta Chim. Sinica 2014, 72,1175. (in Chinese). |
| [25] | ( 高珍珍, 佟浩, 陈建慧, 岳世鸿, 白文龙, 张校刚, 潘燕飞, 石明, 宋玉翔, 化学学报, 2014, 72,1175.) |
| [26] | Jia, W.; Xu, M.-W.; Lei, C.; Bao, S.-J.; Jia, D.-Z. Acta Chim. Sinica 2011, 69,1773. (in Chinese). |
| [26] | ( 贾巍, 徐茂文, 雷超, 包淑娟, 贾殿赠, 化学学报, 2011, 69,1773.) |
| [27] | Kang, Y. J.; Chun, S. J.; Lee, S. S.; Kim, B. Y.; Kim, J. H.; Chung, H.; Lee, S. Y.; Kim, W. ACS Nano 2012, 6,6400. |
| [28] | Wang, H.; Zhu, E.; Yang, J.; Zhou, P.; Sun, D.; Tang, W. J. Phys. Chem. C 2012, 116,13013. |
| [29] | Liu, M.; Miao, Y. E.; Zhang, C.; Tjiu, W. W.; Yang, Z.; Peng, H.; Liu, T. Nanoscale 2013, 5,7312. |
| [30] | Meng, Y.; Wang, K.; Zhang, Y.; Wei, Z. Adv. Mater. 2013, 25,6985. |
| [31] | Rashidi, M.; Tavasoli, A. J. Supercrit. Fluid. 2015, 98,111. |
| [32] | Chen, M. L.; Oh, W. C. Nanoscale Res. Lett. 2011, 6,398. |
| [33] | Hu, W.; Chen, S.; Yang, Z.; Liu, L.; Wang, H. J. Phys. Chem. B 2011, 115,8453. |
| [34] | Mo, Z.-L.; Zhao, Z.-L.; Chen, H.; Niu, G.-P.; Shi, H.-F. Carbohydr. Polym. 2009, 75,660. |
| [35] | Cong, H.-P.; Ren, X.-C.; Wang, P.; Yu, S.-H. Energy Environ. Sci. 2013, 6,1185. |
| [36] | Kumar, A.; Kumar, V.; Kumar, M.; Awasthi, K. Polym. Compos. 2018, 39,3858. |
| [37] | Li, S.; Huang, D.; Zhang, B.; Xu, X.; Wang, M.; Yang, G.; Shen, Y. Adv. Energy Mater. 2014, 4,1301655. |
| [38] | Wu, J.; Zhang, Q. e.; Wang, J.; Huang, X.; Bai, H. Energy Environ. Sci. 2018, 11,1280. |
| [39] | Han, J.; Wang, K.; Liu, W.; Li, C.; Sun, X.; Zhang, X.; An, Y.; Yi, S.; Ma, Y. Nanoscale 2018, 10,13083. |
| [40] | Zang, X.; Li, X.; Zhu, M.; Li, X.; Zhen, Z.; He, Y.; Wang, K.; Wei, J.; Kang, F.; Zhu, H. Nanoscale 2015, 7,7318. |
| [41] | Yu, P.; Zhang, Z.; Zheng, L.; Teng, F.; Hu, L.; Fang, X. Adv. Energy Mater. 2016, 6,1601111. |
| [42] | Yi, H.; Ma, Y.; Zhang, S.; Na, B.; Zeng, R.; Zhang, Y.; Lin, C. ACS Sustainable Chem. Eng. 2019, 7,18894. |
/
| 〈 |
|
〉 |