

基于原位聚合凝胶电解质的碳纳米笼//三氧化钨纳米棒超级电容器
收稿日期: 2021-03-11
网络出版日期: 2021-05-18
基金资助
国家自然科学基金(21972061); 国家自然科学基金(21832003); 国家自然科学基金(21773111); 中央高校基本科研业务费专项资金(14380237); 南京大学博士研究生创新研究项目(CXYJ21-38)
Carbon Nanocages//Tungsten Trioxide Nanorods Supercapacitors with in situ Polymerized Gel Electrolytes
Received date: 2021-03-11
Online published: 2021-05-18
Supported by
National Natural Science Foundation of China(21972061); National Natural Science Foundation of China(21832003); National Natural Science Foundation of China(21773111); Fundamental Research Funds for the Central Universities(14380237); Nanjing University Innovation Program for PhD candidate(CXYJ21-38)
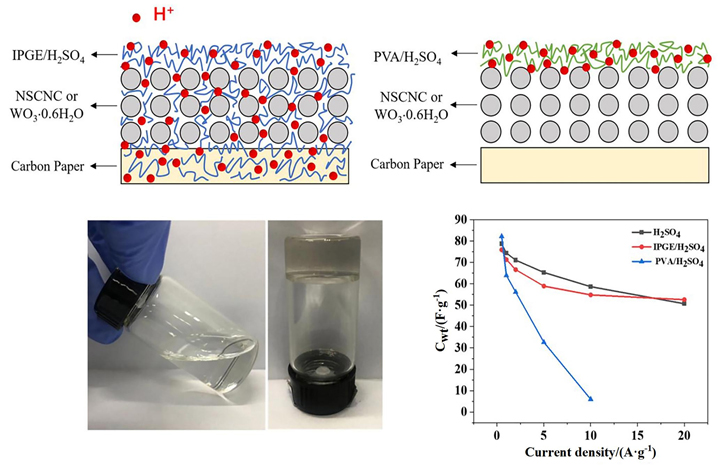
发展非对称超级电容器可有效提升超级电容器能量密度, 选择电极材料和电解质是关键. 分级结构碳纳米笼因具有比表面积大、微孔-介孔-大孔共存、导电性好、稳定性高等优点, 特别适合用作超级电容器电极材料. 进一步通过N, S共掺杂引入赝电容、改善浸润性, 所得的氮硫共掺杂碳纳米笼(NSCNC)在1 mol?L-1 H2SO4溶液、电势范围0~1 V、电流密度1 A?g-1下表现出337 F?g-1的高比容量. 水合三氧化钨(WO3?0.6H2O)纳米棒通过W6+/W5+的氧化还原反应实现H+的嵌入与脱出, 在–0.55~0.3 V、5 A?g-1下表现出454 F?g-1的高比容量. 以NSCNC和WO3?0.6H2O作正负极材料、原位聚合高分子凝胶电解质(IPGE/H2SO4)作准固态电解质组装的非对称超级电容器的工作电压为1.5 V, 其倍率性能非常接近于在H型电解池中以1 mol?L-1 H2SO4为电解液的器件, 而远优于以传统聚乙烯醇/硫酸(PVA/H2SO4)作凝胶电解质的器件, 其根源是原位聚合的IPGE/H2SO4与电极材料之间建立了有效的电荷传输界面, 改善了H+离子的传导, 有效降低了电压降. 本工作不仅展示了酸性介质中NSCNC//WO3?0.6H2O超级电容器的优异储能性能, 还提供了一种新的用于构建准固态超级电容器的原位聚合凝胶电解质.
高润洲 , 李国昌 , 陈轶群 , 曾誉 , 赵杰 , 吴强 , 杨立军 , 王喜章 , 胡征 . 基于原位聚合凝胶电解质的碳纳米笼//三氧化钨纳米棒超级电容器[J]. 化学学报, 2021 , 79(6) : 755 -762 . DOI: 10.6023/A21030087
Asymmetric supercapacitors can effectively increase the energy density of supercapacitors, and the key is to develop high-performance electrode materials and electrolytes. Hierarchical carbon nanocages are promising electrode materials for supercapacitors because of the large specific surface area, coexisting micro-meso-macropore, good conductivity and high stability. By introducing pseudocapacitance and improving wettability via N&S dual-doping, the N&S dual-doped carbon nancages (NSCNC) exhibit a high specific capacity of 337 F?g-1 at 1 A?g-1 within the potential window of 0~1 V in 1 mol?L-1 H2SO4solution. Hydrated tungsten trioxide (WO3?0.6H2O) nanorods can achieve the insertion/de-insertion of H+ via the redox reaction between W6+/W5+, and exhibit a high specific capacity of 454 F?g-1 at 5 A?g-1 within the potential window of –0.55~0.3 V. The solid-state asymmetric supercapacitor (SASC) with NSCNC and WO3?0.6H2O nanorods as positive and negative electrodes, and in situ polymerized gel electrolyte (IPGE/H2SO4) as solid electrolyte has the working voltage of 1.5 V. And the rate performance of the SASC is very close to that of the H-type device with 1 mol?L-1 H2SO4 solution as electrolyte, much better than the counterpart with the traditional gel electrolyte of polyvinyl alcohol/sulfuric acid (PVA/H2SO4). The much improved SASC performance is attributed to the establishment of the effective charge transfer interface between the IPGE/H2SO4 and the electrode materials, which enhances the transfer of H+ ions and thereby much reduces theIR drop. The energy density of the SASC with IPGE/H2SO4 is 22.31 Wh?kg-1 at 0.375 kW?kg-1 (0.5 A?g-1) and 8.55 Wh?kg-1 at 7.5 kW?kg-1 (10 A?g-1), locating at the top-ranking of literatures. The SASC also exbibits excellent cycling stability, with the capacity retention of 95.2% after 4000 cycles at 5 A?g-1. This study not only demonstrates the excellent energy storage performance of NSCNC//WO3?0.6H2O supercapacitors in acidic electrolytes, but also provides a new in-situ polymerized gel electrolyte for building high-performance SASCs.

| [1] | Raza, W.; Ali, F. Z.; Raza, N.; Luo, Y. W.; Kim, K. H.; Yang, J. H.; Kumar, S.; Mehmood, A.; Kwon, E. E. Nano Energy 2018, 52, 441. |
| [2] | Su, S. J.; Lai, Q. X.; Liang, Y. Y. Acta Chim. Sinica 2015, 73, 735. |
| [2] | (苏善金, 来庆学, 梁彦瑜, 化学学报, 2015, 73, 735.) |
| [3] | Muzaffar, A.; Ahamed, M. B.; Deshmukh, K.; Thirumalai, J. Renew. Sust. Energy Rev. 2019, 101, 123. |
| [4] | Zhu, J.; Tang, S. C.; Wu, J.; Shi, X. L.; Zhu, B. G.; Meng, X. K. Adv. Energy Mater. 2017, 7, 1601234. |
| [5] | Couly, C.; Alhabeb, M.; Van Aken, K. L.; Kurra, N.; Gomes, L.; Navarro-Suarez, A. M.; Anasori, B.; Alshareef, H. N.; Gogotsi, Y. Adv. Electron. Mater. 2018, 4, 1700339. |
| [6] | Zhao, J.; Andrew, F. B. Energy Storage Mater. 2021, 36, 31. |
| [7] | Shao, Y.; El-Kady, M. F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R. B. Chem. Rev. 2018, 118, 9233. |
| [8] | Wu, Q.; Yang, L.; Wang, X.; Hu, Z. Adv. Mater. 2020, 32, 1904177. |
| [9] | Yang, L. J.; Shui, J. L.; Du, L.; Shao, Y. Y.; Liu, J.; Dai, L. M.; Hu, Z. Adv. Mater. 2019, 31, 1804799. |
| [10] | Wu, Q.; Yang, L.; Wang, X.; Hu, Z. Sci. China Chem. 2020, 63, 665. |
| [11] | Wu, Q.; Yang, L.; Wang, X.; Hu, Z. Acc. Chem. Res. 2017, 50, 435. |
| [12] | Li, G.; Mao, K.; Liu, M.; Yan, M.; Zhao, J.; Zeng, Y.; Yang, L.; Wu, Q.; Wang, X.; Hu, Z. Adv. Mater. 2020, 32, 2004632. |
| [13] | Zhao, J.; Lai, H.; Lyu, Z.; Jiang, Y.; Xie, K.; Wang, X.; Wu, Q.; Yang, L.; Jin, Z.; Ma, Y.; Liu, J.; Hu, Z. Adv. Mater. 2015, 27, 3541. |
| [14] | Xie, K.; Qin, X.; Wang, X.; Wang, Y.; Tao, H.; Wu, Q.; Yang, L.; Hu, Z. Adv. Mater. 2012, 24, 347. |
| [15] | Jiang, H.; Hong, J. J.; Wu, X.; Surta, T. W.; Qi, Y.; Dong, S.; Li, Z.; Leonard, D. P.; Holoubek, J. J.; Wong, J. C.; Razink, J. J.; Zhang, X.; Ji, X. J. Am. Chem. Soc. 2018, 140, 11556. |
| [16] | Anothumakkool, B.; Torris, A. A. T.; Veeliyath, S.; Vijayakumar, V.; Badiger, M. V.; Kurungot, S. ACS Appl. Mater. Interfaces 2016, 8, 1233. |
| [17] | Lu, X. H.; Yu, M. H.; Wang, G. M.; Tong, Y. X.; Li, Y. Energy Environ. Sci. 2014, 7, 2160. |
| [18] | Wang, F. X.; Wu, X. W.; Yuan, X. H.; Liu, Z. C.; Zhang, Y.; Fu, L. J.; Zhu, Y. S.; Zhou, Q. M.; Wu, Y. P.; Huang, W. Chem. Soc. Rev. 2017, 46, 6816. |
| [19] | Zhu, M.; Wu, J. X.; Wang, Y.; Song, M. M.; Long, L.; Siyal, S. H.; Yang, X. P.; Sui, G. J. Energy Chem. 2019, 37, 126. |
| [20] | Anothumakkool, B.; Torris, A. A. T.; Bhange, S. N.; Unni, S. M.; Badiger, M. V.; Kurungot, S. ACS Appl. Mater. Interfaces 2013, 5, 13397. |
| [21] | Niu, Y. B.; Yin, Y. X.; Wang, W. P.; Wang, P. F.; Guo, Y. G. CCS Chem. 2019, 1, 589. |
| [22] | Fan, H.; Wang, Y.; Gao, F.; Yang, L.; Liu, M.; Du, X.; Wang, P.; Yang, L.; Wu, Q.; Wang, X.; Hu, Z. J. Energy Chem. 2019, 34, 64. |
| [23] | Augustyn, V.; Simon, P.; Dunn, B. Energy Environ. Sci. 2014, 7, 1597. |
| [24] | Lu, S. Y.; Jin, M.; Zhang, Y.; Niu, Y. B.; Gao, J. C.; Li, C. M. Adv. Energy Mater. 2018, 8, 1702545. |
| [25] | Luo, J.; Jang, H. D.; Huang, J. ACS Nano 2013, 7, 1464. |
| [26] | Yan, J.; Fan, Z.; Sun, W.; Ning, G.; Wei, T.; Zhang, Q.; Zhang, R.; Zhi, L.; Wei, F. Adv. Funct. Mater. 2012, 22, 2632. |
| [27] | Zhang, S. W.; Yin, B. S.; Wang, Z. B.; Peter, F. Chem. Eng. J. 2016, 306, 193. |
| [28] | Subramani, K.; Sudhan, N.; Divya, R.; Sathish, M. RSC Adv. 2017, 7, 6648. |
| [29] | Wang, C. Q.; Qiu, F. L.; Deng, H.; Zhang, X. Y.; He, P.; Zhou, H. S. Acta Chim. Sinica 2017, 75, 241. |
| [29] | (王超强, 邱飞龙, 邓瀚, 张晓禹, 何平, 周豪慎, 化学学报, 2017, 75, 241.) |
| [30] | Fan, H. L.; Niu, R. T.; Duan, J. Q.; Liu, W.; Shen, W. Z. ACS Appl. Mater. Interfaces 2016, 8, 19475. |
| [31] | Liu, W. W.; Li, X.; Zhu, M. H.; He, X. J. Power Sources 2015, 282, 179. |
| [32] | Lai, H.; Wu, Q.; Zhao, J.; Shang, L.; Li, H.; Che, R.; Lyu, Z.; Xiong, J.; Yang, L.; Wang, X. Energy Environ. Sci. 2016, 9, 2053. |
| [33] | Gao, D.; Liu, R.; Yu, W.; Luo, Z.; Liu, C.; Fan, S. J. Phys. Chem. C 2019, 123, 5249. |
/
| 〈 |
|
〉 |