

铱催化中氮茚衍生物的Friedel-Crafts类型不对称烯丙基取代反应
收稿日期: 2021-05-08
网络出版日期: 2021-06-11
基金资助
国家自然科学基金(21821002); 国家自然科学基金(21961132002); 上海市科学技术委员会(19590750400)
Ir-Catalyzed Enantioselective Friedel-Crafts Type Allylic Substitution of Indolizines
Received date: 2021-05-08
Online published: 2021-06-11
Supported by
National Natural Science Foundation of China(21821002); National Natural Science Foundation of China(21961132002); Science and Technology Commission of Shanghai Municipality(19590750400)
杨普苏 , 刘晨旭 , 张文文 , 游书力 . 铱催化中氮茚衍生物的Friedel-Crafts类型不对称烯丙基取代反应[J]. 化学学报, 2021 , 79(6) : 742 -746 . DOI: 10.6023/A21050198
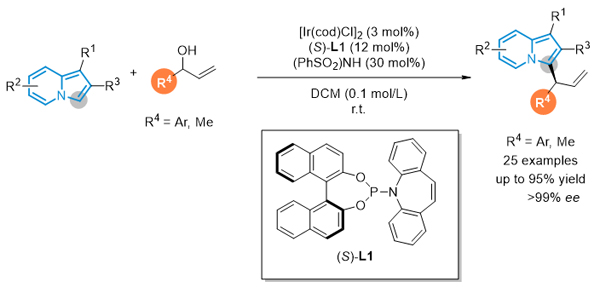
Indolizine is an important N-containing heterocyclic nucleus and their derivatives display interesting biological activities. The development of synthetic methods towards indolizine derivatives has attracted extensive attention. Despite the rapid growth in this area, asymmetric synthesis of indolizine derivatives has been rarely reported. Ir-catalyzed asymmetric allylic substitution reaction is an efficient method for the enantioselective construction of C-C or C-X bonds, furnishing terminal olefins bearing a stereogenic center at the allylic position with excellent regio- and enantioselectivities. In this regard, various electron-rich arenes, including indoles, pyrroles, naphthols, have been applied as the nucleophiles via Friedel-Crafts type process in the allylic substitution reactions. As a class of constitutional isomers of indoles, indolizines are now demonstrated as suitable nucleophiles in transition-metal-catalyzed allylic substitution reactions. Herein, an iridium- catalyzed enantioselective allylic substitution of allylic alcohol with indolizine is reported. A broad range of 3-allylindolizines were accessed with high yields (83%~95%) and excellent enantioselectivities (95%~>99%ee). A general procedure for the asymmetric allylation of indolizines is described in the following: A flame-dried Schlenk tube was cooled to room temperature and filled with argon. To this flask were added [Ir(cod)Cl]2 (4.0 mg, 0.006 mmol, 3 mol%) and (S)-L1 (12.1 mg, 0.024 mmol, 12 mol%). After the flask was evacuated and refilled with argon for three times, freshly distilled dichloromethane (DCM) (2 mL) were added. After the mixture was stirred for 15 min at room temperature, indolizines (0.2 mmol, 1.0 equiv.), allylic alcohols (0.4 mmol, 2.0 equiv.) and (PhSO2)2NH (18.0 mg, 0.06 mmol, 30 mol%) were added under argon atmosphere. The reaction mixture was stirred at room temperature and monitored by thin-layer chromatography (TLC). Once indolizines were consumed, the reaction was diluted with water (5 mL), and the mixture was extracted with EtOAc (10 mL×3). The organic layer was collected, dried over Na2SO4 and then concentrated in vacuo to afford the crude product. This crude material was purified by column chromatography to afford the product.
| [1] | Sharma, V.; Kumar, V. Med. Chem. Res. 2014, 23, 3593. |
| [2] | Wall, M. E.; Wani, M. C.; Cook, C. E.; Palmer, K. H.; Mcphail, A. T.; Sim, G. A. J. Am. Chem. Soc. 1966, 16, 3888. |
| [3] | (a) Gundersen, L.-L.; Negussie, A. H.; Rise, F.; ?stby, O. B. Arch. Pharm. 2003, 336, 191. |
| [3] | (b) Huang, W.; Zuo, T.; Jin, H.; Liu, Z.; Yang, Z.; Yu, X.; Zhang, L.; Zhang, L. Mol. Diversity 2013, 17, 221. |
| [3] | (c) Xue, Y.; Tang, J.; Ma, X.; Li, Q.; Xie, B.; Hao, Y.; Jin, H.; Wang, K.; Zhang, G.; Zhang, L.; Zhang, L. Eur. J. Med. Chem. 2016, 115, 94. |
| [3] | (d) Park, S.; Kim, E. H.; Kim, J.; Kim, S. H.; Kim, I. Eur. J. Med. Chem. 2018, 144, 435. |
| [4] | (a) Katritzky, A. R.; Qiu, G.; Yang, B.; He, H.-Y. J. Org. Chem. 1999, 64, 7618. |
| [4] | (b) Zhang, L.; Liang, F.; Sun, L.; Hu, Y.; Hu, H. Synthesis 2000, 12, 1733. |
| [4] | (c) Bora, U.; Saikia, A.; Boruah, R. C. Org. Lett. 2003, 5, 435. |
| [4] | (d) Fang, X.; Wu, Y.-M.; Deng, J.; Wang, S.-W. Tetrahedron 2004, 60, 5487. |
| [4] | (e) Rotaru, A. V.; Druta, I. D.; Oeser, T.; Muller, T. J. J. Helv. Chim. Acta 2005, 88, 1798. |
| [4] | (f) Smith, C. R.; Bunnelle, E. M.; Rhodes, A. J.; Sarpong, R. Org. Lett. 2007, 9, 1169. |
| [4] | (g) Xia, J.-B.; You, S.-L. Org. Lett. 2009, 11, 1187. |
| [4] | (h) Muthusaravanan, S.; Perumal, S.; Yogeeswari, P.; Sriram, D. Tetrahedron Lett. 2010, 51, 6439. |
| [4] | (i) Zhu, H.; Stockigt, J.; Yu, Y.; Zou, H. Org. Lett. 2011, 13, 2792. |
| [4] | (j) Jung, Y.; Kim, I. Tetrahedron 2012, 68, 8198. |
| [4] | (k) Lee, J. H.; Kim, I. J. Org. Chem. 2013, 78, 1283. |
| [4] | (l) Liang, Y.; Teng, L.; Wang, Y.; He, Q.; Cao, H. Green Chem. 2019, 21, 4025. |
| [4] | (m) Silva, T. S.; Zeoly, L. A.; Coelho, F. J. Org. Chem. 2020, 85, 5438. |
| [4] | (n) Guidotti, B. B.; Silva, T. S. D.; Correia, J. T. M.; Coelho, F. Org. Biomol. Chem. 2020, 18, 7330. |
| [5] | (a) Jana, R.; Pathak, T. P.; Jensen, K. H.; Sigman, M. S. Org. Lett. 2012, 14, 4074. |
| [5] | (b) Correia, J. T. M.; List, B.; Coelho, F. Angew. Chem., Int. Ed. 2017, 56, 7967. |
| [5] | (c) Chen, H.; Zhu, L.; Zhong, K.; Yue, X.; Qu, L.-B.; Bai, R.; Lan, Y. Chin. Chem. Lett. 2018, 29, 1237. |
| [5] | (d) Yang, P.-J.; Qi, L.; Liu, Z.; Yang, G.; Chai, Z. J. Am. Chem. Soc. 2018, 140, 17211. |
| [5] | (e) Yang, L.; Pu, X.; Niu, D.; Fu, Z.; Zhang, X. Org. Lett. 2019, 21, 8553. |
| [5] | (f) Li, K.; Li, C. Org. Lett. 2020, 22, 9456. |
| [6] | (a) Hartwig, J. F.; Stanley, L. M. Acc. Chem. Res. 2010, 43, 1461. |
| [6] | (b) Qu, J.; Helmchen, G. Acc. Chem. Res. 2017, 50, 2539. |
| [6] | (c) Deng, Y.; Yang, W.; Yang, X.; Yang, D. Chin. J. Org. Chem. 2017, 37, 3039. (in Chinese) |
| [6] | (邓颖颍, 杨文, 杨新, 杨定乔, 有机化学, 2017, 37, 3039.) |
| [6] | (d) Cheng, Q.; Tu, H.-F.; Zheng, C.; Qu, J.-P.; Helmchen, G.; You, S.-L. Chem. Rev. 2019, 119, 1855. |
| [6] | (e) Ro?ssler, S. L.; Petrone, D. A.; Carreira, E. M. Acc. Chem. Res. 2019, 52, 2657. |
| [6] | (f) Tian, F.; Zhang, J.; Yang, W.; Deng, W. Chin. J. Org. Chem. 2020, 40, 3262. (in Chinese) |
| [6] | (田飞, 张键, 杨武林, 邓卫平, 有机化学, 2020, 40, 3262.) |
| [7] | (a) Janssen, J. P.; Helmchen, G. Tetrahedron Lett. 1997, 38, 8025. |
| [7] | (b) Alexakis, A.; Polet, D. Org. Lett. 2004, 6, 3529. |
| [7] | (c) Streiff, S.; Welter, C.; Schelwies, M.; Lipowsky, G.; Miller, N.; Helmchen, G. Chem. Commun. 2005,2957. |
| [7] | (d) Chen, W.; Hartwig, J. F. J. Am. Chem. Soc. 2013, 135, 2068. |
| [7] | (e) Liang, X.; Wei, K.; Yang, Y.-R. Chem. Commun. 2015, 51, 17471. |
| [7] | (f) Jiang, X.; Boehm, P.; Hartwig, J. F. J. Am. Chem. Soc. 2018, 140, 1239. |
| [7] | (g) Huo, X.; Zhang, J.; Fu, J.; He, R.; Zhang, W. J. Am. Chem. Soc. 2018, 140, 2080. |
| [7] | (h) Sempere, Y.; Carreira, E. M. Angew. Chem., Int. Ed. 2018, 57, 7654. |
| [8] | (a) Ohmura, T.; Hartwig, J. F. J. Am. Chem. Soc. 2002, 124, 15164. |
| [8] | (b) Lo?pez, F.; Ohmura, T.; Hartwig, J. F. J. Am. Chem. Soc. 2003, 125, 3426. |
| [8] | (c) Lipowsky, G.; Helmchen, G. Chem. Commun. 2004,116. |
| [8] | (d) Shu, C.; Leitner, A.; Hartwig, J. F. Angew. Chem., Int. Ed. 2004, 43, 4797. |
| [8] | (e) Tissot-Croset, K.; Polet, D.; Alexakis, A. Angew. Chem., Int. Ed. 2004, 43, 2426. |
| [8] | (f) Fischer, C.; Defieber, C.; Suzuki, T.; Carreira, E. M. J. Am. Chem. Soc. 2004, 126, 1628. |
| [8] | (g) Roggen, M.; Carreira, E. M. J. Am. Chem. Soc. 2010, 132, 11917. |
| [8] | (h) Stanley, L. M.; Bai, C.; Ueda, M.; Hartwig, J. F. J. Am. Chem. Soc. 2010, 132, 8918. |
| [8] | (i) Roggen, M.; Carreira, E. M. Angew. Chem., Int. Ed. 2011, 50, 5568. |
| [9] | (a) Liu, W.-B.; He, H.; Dai, L.-X.; You, S.-L. Org. Lett. 2008, 10, 1815. |
| [9] | (b) Wu, Q.-F.; He, H.; Liu, W.-B.; You, S.-L. J. Am. Chem. Soc. 2010, 132, 11418. |
| [9] | (c) Huang, L.; Dai, L.-X.; You, S.-L. J. Am. Chem. Soc. 2016, 138, 5793. |
| [9] | (d) Huang, L.; Cai, Y.; Zhang, H.-J.; Dai, L.-X.; You, S.-L. CCS Chem. 2019, 1, 106. |
| [9] | (e) Zhang, J.; Gao, Y.-S.; Gu, B.-M.; Yang, W.-L.; Tian, B.-X.; Deng, W.-P. ACS Catal. 2021, 11, 3810. |
| [9] | (f) Jiang, R.; Ding, L.; Zheng, C.; You, S.-L. Science 2021, 371, 380. |
| [9] | (g) Jiang, R.; Zheng, C.; You, S.-L. Chin. Sci. Bull. doi: 10.1360/TB-2021-0096 (in Chinese) |
| [9] | (蒋茹, 郑超, 游书力, 科学通报, doi: 10.1360/TB-2021-0096.) |
| [10] | (a) Zhuo, C.-X.; Cheng, Q.; Liu, W.-B.; Zhao, Q.; You, S.-L. Angew. Chem., Int. Ed. 2015, 54, 8475. |
| [10] | (b) Huang, L.; Cai, Y.; Zheng, C.; Dai, L.-X.; You, S.-L. Angew. Chem., Int. Ed. 2017, 56, 10545. |
| [11] | (a) Wu, Q.-F.; Liu, W.-B.; Zhuo, C.-X.; Rong, Z.-Q.; Ye, K.-Y.; You, S.-L. Angew. Chem., Int. Ed. 2011, 50, 4455. |
| [11] | (b) Nemoto, T.; Ishige, Y.; Yoshida, M.; Kohno, Y.; Kanematsu, M.; Hamada, Y. Org. Lett. 2010, 12, 5020. |
| [11] | (c) Yoshida, M.; Nemoto, T.; Zhao, Z.; Ishige, Y.; Hamada, Y. Tetrahedron: Asymmetry 2012, 23, 859. |
| [11] | (d) Xu, Q.-L.; Dai, L.-X.; You, S.-L. Org. Lett. 2012, 14, 2579. |
| [11] | (e) Cheng, Q.; Wang, Y.; You, S.-L. Angew. Chem., Int. Ed. 2016, 55, 3496. |
| [12] | For a book: You, S.-L;. Asymmetric Dearomatization Reactions, Wiley-VCH, Weinheim, Germany, 2016. |
| [12] | For reviews: (a) Ding, Q.; Zhou, X.; Fan, R;. Org. Biomol. Chem. 2014, 12, 4807. |
| [12] | (b) Zhuo, C.-X.; Zheng, C.; You, S.-L. Acc. Chem. Res. 2014, 47, 2558. |
| [12] | (c) Wu, W.-T.; Zhang, L.; You, S.-L. Chem. Soc. Rev. 2016, 45, 1570. |
| [12] | (d) Zheng, C.; You, S.-L. Chem 2016, 1, 830. |
| [12] | (e) Wu, W.-T.; Zhang, L.; You, S.-L. Acta Chim. Sinica 2017, 75, 419. (in Chinese) |
| [12] | (吴文挺, 张立明, 游书力, 化学学报, 2017, 75, 419.) |
| [12] | (f) Zhu, M.; Zhang, X.; You, S.-L. Chem. J. Chin. Univ. 2020, 41, 1407. (in Chinese) |
| [12] | (朱敏, 张霄, 游书力, 高等学校化学学报, 2020, 41, 1407.) |
| [13] | During the preparation of our manuscript, Zeng and co-workers reported an iridium catalyzed asymmetric allylic substitution reaction of indolizine derivatives, see: Lu, J.; Wang, M.; Xu, R.; Sun, H.; Zheng, X.; Zhong, G.; Zeng, X.. Asian J. Org. Chem. 2021, 10, 1500. |
| [14] | Defieber, C.; Ariger, M. A.; Moriel, P.; Carreira, E. M. Angew. Chem., Int. Ed. 2007, 46, 3139. |
| [15] | Ro?ssler, S. L.; Krautwald, S.; Carreira, E. M. J. Am. Chem. Soc. 2017, 139, 3603. |
/
| 〈 |
|
〉 |