

哌嗪季铵碱催化剂的合成及其在异氰酸酯聚合反应中的应用
收稿日期: 2021-06-29
网络出版日期: 2021-08-02
基金资助
项目受国家自然科学基金(21673117); 项目受国家自然科学基金(91956109); 江苏省特聘教授基金和南京工业大学科研启动基金(39837126); 江苏省特聘教授基金和南京工业大学科研启动基金(39837102)
Synthesis of Piperazine Quaternary Ammonium Alkali Catalyst and Its Application in Isocyanate Polymerization
Received date: 2021-06-29
Online published: 2021-08-02
Supported by
National Natural Science Foundation of China(21673117); National Natural Science Foundation of China(91956109); Jiangsu Provincial Foundation for Specially-Appointed Professor, and Start-up Fund from Nanjing Tech University(39837126); Jiangsu Provincial Foundation for Specially-Appointed Professor, and Start-up Fund from Nanjing Tech University(39837102)
许昕彤 , 谭伟民 , 姬梦圆 , 杨悦 , 饶兴兴 , 雒新亮 , 张延华 , 陈虹宇 . 哌嗪季铵碱催化剂的合成及其在异氰酸酯聚合反应中的应用[J]. 化学学报, 2021 , 79(9) : 1113 -1117 . DOI: 10.6023/A21060300
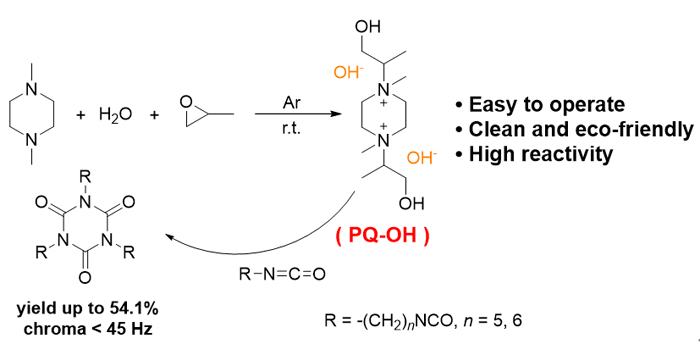
Aliphatic isocyanate trimer has the advantage of excellent thermal stability, radiation resistance, corrosion resistance and flame retardancy. It is an important unit for various chemical products. Hexamethylene diisocyanate trimer (HDIT) is one of the popular trimers, which is mainly synthesized through the polymerization of monomers. A variety of catalysts have been applied in this procedure, including metal salts, phosphines, aminosilanes, N-heterocyclic carbenes and N-heterocyclic olefins. However, HDI monomer is usually expensive, while low catalyst activity, harsh preparation conditions, and easy-formation of polymer are often observed in the polymerization process. Pentamethylene diisocyanate (PDI), a cheap bio-based linear aliphatic polyisocyanate with similar structure to traditional HDI, has the potential to replace HDI. Since the activity of PDI is lower, it is found that the catalysts applicable for HDI work not well for PDI. Herein, we design and synthesize 1,4-bis(2-hydroxy-isopropyl)-1,4-dimethylpiperazine bisquaternary ammonium base (PQ-OH) as the catalyst for the production of both HDI trimer and PDI trimer (PDIT). Due to having two hydroxide ions, PQ-OH catalyst can attack the -NCO groups of two monomers at the same time, thus improving the reaction efficiency. Moreover, the β-OH groups on the catalyst molecule also promote the polymerization process. The piperazine ring structure in the catalyst has obvious steric hindrance effect, which can enhance the reaction between small molecules, and inhibit the reaction between large molecules and small molecules. Thus, the product with high trimer content is obtained. A general synthesis procedure for PQ-OH is described as followed. Propylene epoxide (70.0 mL, 1.0 mol) is added to a mixture of 1,4-dimethylpiperazine (67.6 mL, 0.5 mol) and water (18 mL, 1.0 mol) dropwisely under argon. After stirring at room temperature for 4 h, the solution turns to brown and stratifies. The lower layer is separated and concentrated in vacua to remove excess propylene oxide, water and possible alcohol by-products. The obtained crude product is dissolved in methanol, followed by decolorizing with activated carbon and drying with molecular sieve. After filtering and concentration, the desired product is obtained as a white viscous liquid.
| [1] | (a) He, Y.; Zhang, X.; Zhang, X.; Huang, H.; Chang, J.; Chen, H. J. Ind. Eng. Chem. 2012, 18, 1620; |
| [1] | (b) Giuglio-Tonolo, A. G.; Spitz, C.; Terme, T.; Vanelle, P. Tetrahedron Lett. 2014, 55, 2700; |
| [1] | (c) Driest, P. J.; Lenzi, V.; Marques, L. S. A.; Ramos, M. M. D.; Dijkstra, D. J.; Richter, F. U.; Stamatialis, D.; Grijpma, D. W. Polym. Adv. Technol. 2017, 28, 1299; |
| [1] | (d) Wu, L.; Liu, W.; Ye, J.; Cheng, R. Catal. Commun. 2020, 145, 106097. |
| [2] | Chen, Z. M.S. Thesis, South China University of Technology, Guangzhou, 2014. (in Chinese) |
| [2] | ( 陈卓, 硕士论文, 华南理工大学, 广州, 2014.) |
| [3] | Qiu, S. J.; Gan, X. X.; Lu, X. M. Chem. Adhesion 2001, 04, 165. (in Chinese) |
| [3] | ( 邱少君, 甘孝贤, 卢先明, 化学与粘合 2001, 04, 165.) |
| [4] | Sharpe, H. R.; Geer, A. M.; Williams, H. E.; Blundell, T. J.; Lewis, W.; Blake, A. J.; Kays, D. L. Chem. Commun. 2017, 53, 937. |
| [5] | Helberg, J.; Oe, Y.; Zipse, H. Chemistry 2018, 24, 14387. |
| [6] | Roman, M.; Andrioletti, B.; Lemaire, M.; Bernard, J.-M.; Schwartz, J.; Barbeau, P. Tetrahedron 2011, 67, 1506. |
| [7] | Duong, H. A.; Cross, M. J.; Louie, J. Org. Lett. 2004, 6, 4679. |
| [8] | Li, C.; Zhao, W.; He, J.; Zhang, Y. Chem. Commun. 2019, 55, 12563. |
| [9] | Hsieh, K. H.; Kresta, J. E. In ACS Symposium Series, Vol. 195, Ed.: George, B.; Butler, E., Kresta, America, 1982, Chapter 24, p. 311. |
| [10] | Tan, W. M.; Wang, L.; Di, Z. G.; Luo, X. L.; Shi, L. P.; Wang, Y. X.; Yu, F.; Xu, X.; Chen, K. Q. PCI 2020, 50, 38. (in Chinese) |
| [10] | ( 谭伟民, 王黎, 狄志刚, 雒新亮, 史立平, 王亚鑫, 郁飞, 许旭, 陈可泉, 涂料工业, 2020, 50, 38.) |
| [11] | Qiu, S. J.; Gan, X. X.; Wang, J. G.; Yang, Y. PUI 1998, 02, 17. (in Chinese) |
| [11] | ( 邱少君, 甘孝贤, 王吉贵, 杨毅, 聚氨酯工业, 1998, 02, 17.) |
| [12] | Luo, X. L.; Di, Z. G.; Tan, W. M.; Shi, L. P.; Wang, L.; Wang, Y. X. PCI 2020, 50, 1. (in Chinese) |
| [12] | ( 雒新亮, 狄志刚, 谭伟民, 史立平, 王黎, 王亚鑫, 涂料工业, 2020, 50, 1.) |
| [13] | Morales-Cerrada, R.; Tavernier, R.; Caillol, S. Polymers 2021, 13, 1255. |
| [14] | Siebert, M.; Sure, R.; Deglmann, P.; Closs, A. C.; Lucas, F.; Trapp, O. J. Org. Chem. 2020, 85, 8553. |
| [15] | Peters, S. J.; Klen, J. R.; Smart, N. S. Org. Lett. 2008, 10, 4521. |
/
| 〈 |
|
〉 |