

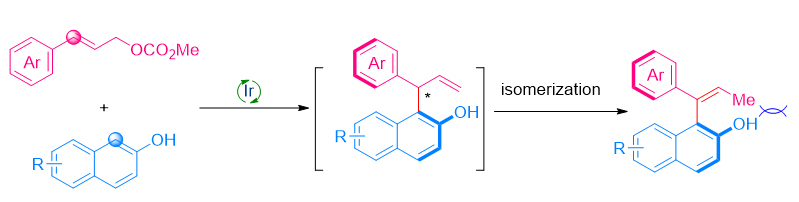
铱催化串联不对称烯丙基取代/双键异构化构建轴手性化合物
收稿日期: 2021-07-11
网络出版日期: 2021-08-17
基金资助
项目受国家自然科学基金(21821002); 项目受国家自然科学基金(21961132002); 上海市科学技术委员会(19590750400)
Ir-catalyzed Sequential Asymmetric Allylic Substitution/Olefin Isomerization for the Synthesis of Axially Chiral Compounds
Received date: 2021-07-11
Online published: 2021-08-17
Supported by
National Natural Science Foundation of China(21821002); National Natural Science Foundation of China(21961132002); Science and Technology Commission of Shanghai Municipality(19590750400)
赵庆如 , 蒋茹 , 游书力 . 铱催化串联不对称烯丙基取代/双键异构化构建轴手性化合物[J]. 化学学报, 2021 , 79(9) : 1107 -1112 . DOI: 10.6023/A21070320
Axially chiral compounds represent an important class of chiral molecules. In this regard, many methods have been developed to access these compounds. However, efficient methods for the synthesis of axially chiral styrenes are limited to date, mainly due to their relative instability compared to axially chiral biaryl compounds. Iridium-catalyzed asymmetric allylic substitutions have evolved as a powerful tool in constructing C―C or C―X bonds at the allylic position. We developed an efficient sequential strategy to access a series of axially chiral styrenes by iridium-catalyzed allylic substitution and central-to-axial chirality transfer via olefin isomerization. With the iridium complex derived from [Ir(cod)Cl]2 and Alexakis ligand L1 as catalyst, and β-naphthol as nucleophiles, a broad range of axially chiral styrenes were obtained with moderate to excellent yields (28%~97% yields) and enantioselectivity (59%~98% ee). A general procedure for the asymmetric allylation of β-naphthol is described as the following: A flame-dried Schlenk tube was cooled to room temperature and filled with argon. To this flask were added [Ir(cod)Cl]2 (2.6 mg, 0.004 mmol, 2 mol%), (S,S,Sa)-L1 (4.8 mg, 0.008 mmol, 4 mol%), freshly distilled tetrahydrofuran (THF, 0.5 mL) and propylamine (0.5 mL). The mixture was stirred at 50 ℃ for 30 min and then the low-boiling solvents were removed in vacuo to give a pale yellow solid. The solid was stirred at 50 ℃ again under vacuum until it became a powder. After that, β-naphthol 1 (0.22 mmol, 1.1 equiv.), allyl carbonate 2 (0.2 mmol, 1.0 equiv.), 1,4-diazobicyclo(2.2.2)octane (DABCO) (67.3 mg, 0.6 mmol, 3.0 equiv.) and freshly distilled Et2O (2.0 mL) were added to this flask under argon atmosphere. The reaction mixture was stirred at 20 ℃ until the starting material was consumed (monitored by thin layer chromatography, TLC). The crude reaction mixture was diluted with water (5 mL), and extracted with dichloromethane (DCM, 5 mL×3). The organic layers were collected, dried over Na2SO4 and then concentrated in vacuo to afford the crude product. The residue was purified by preparative TLC to afford the product.
| [1] | For reviews: (a) Kumarasamy, E.; Raghunathan, R.. Sibi, M. P.. Sivaguru, J. Chem. Rev. 2015, 115, 11239. |
| [1] | (b) Wang, Y.-B.; Tan, B. Acc. Chem. Res. 2018, 51, 534. |
| [1] | (c) Zhang, S.; Liao, G.; Shi, B. Chin. J. Org. Chem. 2019, 39, 1522. (in Chinese) |
| [1] | ( 张硕, 廖港, 史炳锋, 有机化学, 2019, 39, 1522.) |
| [1] | (d) Cheng, J. K.; Xiang, S.-H.; Li, S.; Ye, L.; Tan, B. Chem. Rev. 2021, 121, 4805. |
| [2] | For reviews: (a) Kozlowski, M. C.; Morgan, B. J.; Linton, E. C. Chem. Soc. Rev. 2009, 38, 3193. |
| [2] | (b) Bringmann, G.; Gulder, T.; Gulder, T. A.; Breuning, M. Chem. Rev. 2011, 111, 563. |
| [2] | (c) Erbas-Cakmak, S.; Leigh, D. A.; McTernan, C. T.; Nussbaumer, A. L. Chem. Rev. 2015, 115, 10081. |
| [3] | Miyashita, A.; Yasuda, A.; Takaya, H.; Toriumi, K.; Ito, T.; Souchi, T.; Noyori, R. J. Am. Chem. Soc. 1980, 102, 7932. |
| [4] | For books: (a) Zhou, Q.-L. Privileged Chiral Ligands and Catalysts, Wiley-VCH, Weinheim, Germany, 2011. |
| [4] | (b) You, S.-L. Asymmetric Dearomatization Reactions, Wiley-VCH, Weinheim, Germany, 2016. For review: |
| [4] | (c) Li, Y.-M.; Kwong, F.-Y.; Yu, W.-Y.; Chan, A. S. C. Coord. Chem. Rev. 2007, 251, 2119. |
| [5] | For reviews: (a) Wencel-Delord, J.; Panossian, A.; Leroux, F. R.; Colobert, F. Chem. Soc. Rev. 2015, 44, 3418. |
| [5] | (b) Loxq, P.; Manoury, E.; Poli, R.; Deydier, E.; Labande, A. Coord. Chem. Rev. 2016, 308, 131. |
| [5] | (c) Wang, Q.; Gu, Q.; You, S.-L. Acta Chim. Sinica 2019, 77, 690. (in Chinese) |
| [5] | ( 王强, 顾庆, 游书力, 化学学报, 2019, 77, 690.) |
| [6] | Gu, Z.; Feng, J. SynOpen 2021, 5, 68. |
| [7] | For selected examples: (a) Zheng, S.-C.; Wu, S.; Zhou, Q.; Chung, L. W.; Ye, L.; Tan, B. Nat. Commun. 2017, 8, 15238. |
| [7] | (b) Tan, Y.; Jia, S.; Hu, F.; Liu, Y.; Peng, L.; Li, D.; Yan, H. J. Am. Chem. Soc. 2018, 140, 16893. |
| [7] | (c) Jia, S.; Chen, Z.; Zhang, N.; Tan, Y.; Liu, Y.; Deng, J.; Yan, H. J. Am. Chem. Soc. 2018, 140, 7056. |
| [7] | (d) Wang, C.-S.; Li, T.-Z.; Liu, S.-J.; Zhang, Y.-C.; Deng, S.; Jiao, Y.; Shi, F. Chin. J. Chem. 2020, 38, 543. |
| [7] | (e) Sheng, F.-T.; Li, Z.-M.; Zhang, Y.-Z.; Sun, L.-X.; Zhang, Y.-C.; Tan, W.; Shi, F. Chin. J. Chem. 2020, 38, 583. |
| [7] | (f) Wang, J.-Y.; Sun, M.; Yu, X.-Y.; Zhang, Y.-C.; Tan, W.; Shi, F. Chin. J. Chem. 2021, 39, 2163. |
| [8] | For selected examples: (a) Bringmann, G.; Price Mortimer, A. J.; Keller, P. A.; Gresser, M. J.; Garner, J.; Breuning, M.. Angew. Chem. Int. Ed. 2005, 44, 5384. |
| [8] | (b) Pan, C.; Zhu, Z.; Zhang, M.; Gu, Z. Angew. Chem. Int. Ed. 2017, 56, 4777. |
| [9] | For selected examples: (a) Wang, F.; Qi, Z.; Zhai, S.; Zheng, G.; Mi, R.; Huang, Z.; Zhu, X.; He, X.; Li, X. Angew. Chem. Int. Ed. 2020, 59, 13288. |
| [9] | (b) Yang, C.; Wu, T.-R.; Li, Y.; Wu, B.-B.; Jin, R.-X.; Hu, D.-D.; Li, Y.-B.; Bian, K.-J.; Wang, X.-S. Chem. Sci. 2021, 12, 3726. |
| [10] | Feng, J.; Li, B.; He, Y.; Gu, Z. Angew. Chem. Int. Ed. 2016, 55, 2186. |
| [11] | Sun, C.; Qi, X.; Min, X.-L.; Bai, X.-D.; Liu, P.; He, Y. Chem. Sci. 2020, 11, 10119. |
| [12] | For reviews of allylic substitution reactions: (a) Zhang, M.-M.; Luo, Y.-Y.; Lu, L.-Q.; Xiao, W.-J. Acta Chim. Sinica 2018, 76, 838. (in Chinese) |
| [12] | ( 张毛毛, 骆元元, 陆良秋, 肖文精, 化学学报, 2018, 76, 838.) |
| [12] | (b) Ma, X.; Yu, J.; Wang, Z.; Zhang, Y.; Zhou, Q. Chin. J. Org. Chem. 2020, 40, 2669. (in Chinese) |
| [12] | 马献涛, 于静, 王子龙, 张赟, 周秋菊, 有机化学, 2020, 40, 2669.) |
| [12] | For selected examples of allylic substitution reactions: (c) Yao, K.; Liu, H.; Yuan, Q.; Liu, Y.; Liu, D.; Zhang, W. Acta Chim. Sinica 2019, 77, 993. (in Chinese) |
| [12] | ( 姚坤, 刘浩, 袁乾家, 刘燕刚, 刘德龙, 张万斌, 化学学报, 2019, 77, 993.) |
| [12] | (d) Xiao, J.; Xu, H.; Huo, X.; Zhang, W.; Ma, S. Chin. J. Chem. 2021, 39, 1958. |
| [12] | (e) Huo, X.; Zhao, L.; Luo, Y.; Wu, Y.; Sun, Y.; Li, G.; Gridneva, T.; Zhang, J.; Ye, Y.; Zhang, W. CCS Chem. 2021, 3, 1933. |
| [13] | For reviews of iridium-catalyzed allylic substitution reactions: (a) Hartwig, J. F.; Stanley, L. M.. Acc. Chem. Res. 2010, 43, 1461. |
| [13] | (b) Qu, J.; Helmchen, G. Acc. Chem. Res. 2017, 50, 2539. |
| [13] | (c) Deng, Y.; Yang, W.; Yang, X.; Yang, D. Chin. J. Org. Chem. 2017, 37, 3039. (in Chinese) |
| [13] | ( 邓颖颍, 杨文, 杨新, 杨定乔, 有机化学, 2017, 37, 3039.) |
| [13] | (d) Cheng, Q.; Tu, H.-F.; Zheng, C.; Qu, J.-P.; Helmchen, G.; You, S.-L. Chem. Rev. 2019, 119, 1855. |
| [13] | (e) Tian, F.; Zhang, J.; Yang, W.; Deng, W. Chin. J. Org. Chem. 2020, 40, 3262. (in Chinese) |
| [13] | 田飞, 张键, 杨武林, 邓卫平, 有机化学, 2020, 40, 3262. |
| [14] | For selected examples of iridium-catalyzed allylic substitution reactions: (a) Krautwald, S.; Sarlah, D.; Schafroth, M. A.; Carreira, E. M. Science 2013, 340, 1065. |
| [14] | (b) Liu, W.-B.; Reeves, C. M.; Stoltz, B. M. J. Am. Chem. Soc. 2013, 135, 17298. |
| [14] | (c) Liu, J.; Cao, C.-G.; Sun, H.-B.; Zhang, X.; Niu, D. J. Am. Chem. Soc. 2016, 138, 13103. |
| [14] | (d) Huo, X.; He, R.; Zhang, X.; Zhang, W. J. Am. Chem. Soc. 2016, 138, 11093. |
| [14] | (e) Huo, X.; Zhang, J.; Fu, J.; He, R.; Zhang, W. J. Am. Chem. Soc. 2018, 140, 2080. |
| [14] | (f) Wei, L.; Zhu, Q.; Xu, S.-M.; Chang, X.; Wang, C.-J. J. Am. Chem. Soc. 2018, 140, 1508. |
| [14] | (g) Xu, S.-M.; Wei, L.; Shen, C.; Xiao, L.; Tao, H.-Y.; Wang, C.-J. Nat. Commun. 2019, 10, 5553. |
| [14] | (h) Han, M.; Yang, M.; Wu, R.; Li, Y.; Jia, T.; Gao, Y.; Ni, H.-L.; Hu, P.; Wang, B.-Q.; Cao, P. J. Am. Chem. Soc. 2020, 142, 13398. |
| [14] | (i) Yang, P.; Liu, C.-X.; Zhang, W.-W.; You, S.-L. Acta Chim. Sinica. 2021, 79, 742. (in Chinese) |
| [14] | ( 杨普苏, 刘晨旭, 张文文, 游书力, 化学学报, 2021, 79, 742.) |
| [15] | For selected examples: (a) Liu, W.-B.; He, H.; Dai, L.-X.; You, S.-L. Org. Lett. 2008, 10, 1815. |
| [15] | (b) Wu, Q.-F.; He, H.; Liu, W.-B.; You, S.-L. J. Am. Chem. Soc. 2010, 132, 11418. |
| [15] | (c) Huang, L.; Dai, L.-X.; You, S.-L. J. Am. Chem. Soc. 2016, 138, 5793. |
| [15] | (d) Jiang, S.-Z.; Zeng, X.-Y.; Liang, X.; Lei, T.; Wei, K.; Yang, Y.-R. Angew. Chem. Int. Ed. 2016, 55, 4044. |
| [15] | (e) Tu, H.-F.; Zhang, X.; Zheng, C.; Zhu, M.; You, S.-L. Nat. Catal. 2018, 1, 601. |
| [15] | (f) Huang, L.; Cai, Y.; Zhang, H.-J.; Dai, L.-X.; You, S.-L. CCS Chem. 2019, 1, 106. |
| [15] | (g) Uno, H.; Kawai, K.; Shiro, M.; Shibata, N. ACS Catal. 2020, 10, 14117. |
| [15] | (h) Jiang, R.; Ding, L.; Zheng, C.; You, S.-L. Science 2021, 371, 380. |
| [15] | (i) Zhang, J.; Gao, Y.-S.; Gu, B.-M.; Yang, W.-L.; Tian, B.-X.; Deng, W.-P. ACS Catal. 2021, 11, 3810. |
| [16] | For selected examples: (a) Zhuo, C.-X.; Liu, W.-B.; Wu, Q.-F.; You, S.-L. Chem. Sci. 2012, 3, 205. |
| [16] | (b) Zhuo, C.-X.; Wu, Q.-F; Zhao, Q.; Xu, Q.-L.; You, S.-L. J. Am. Chem. Soc. 2013, 135, 8169. |
| [16] | (c) Zhuo, C.-X.; Cheng, Q.; Liu, W.-B.; Zhao, Q.; You, S.-L. Angew. Chem. Int. Ed. 2015, 54, 8475. |
| [16] | (d) Huang, L.; Cai, Y.; Zheng, C.; Dai, L.-X.; You, S.-L. Angew. Chem. Int. Ed. 2017, 56, 10545. |
| [16] | (e) Zi, Y.; Lange, M.; Schultz, C.; Vilotijevic, I. Angew. Chem. Int. Ed. 2019, 58, 10727. |
| [17] | For selected examples: (a) Bechem, B.; Patman, R. L.; Hashmi, A. S. K.; Krische, M. J. J. Org. Chem. 2010, 75, 1795. |
| [17] | (b) Chen, W.; Hartwig, J. F. J. Am. Chem. Soc. 2012, 134, 15249. |
| [18] | For selected examples: (a) Nemoto, T.; Ishige, Y.; Yoshida, M.; Kohno, Y.; Kanematsu, M.; Hamada, Y. Org. Lett. 2010, 12, 5020. |
| [18] | (b) Wu, Q.-F.; Liu, W.-B.; Zhuo, C.-X.; Rong, Z.-Q.; Ye, K.-Y.; You, S.-L. Angew. Chem., Int. Ed. 2011, 50, 4455. |
| [18] | (c) Xu, Q.-L.; Dai, L.-X.; You, S.-L. Org. Lett. 2012, 14, 2579. |
| [18] | (d) Zhuo, C.-X.; You, S.-L. Angew. Chem., Int. Ed. 2013, 52, 10056. |
| [18] | (e) Cheng, Q.; Wang, Y.; You, S.-L. Angew. Chem., Int. Ed. 2016, 55, 3496. |
| [18] | (f) Tu, H.-F.; Zheng, C.; Xu, R.-Q.; Liu, X.-J.; You, S.-L. Angew. Chem. Int. Ed. 2017, 56, 3237. |
| [18] | (g) Shen, D.; Chen, Q.; Yan, P.; Zeng, X.; Zhong, G. Angew. Chem. Int. Ed., 2017, 56, 3242. |
| [19] | Computational studies of the isomerization process were developed by He and coworkers: Wang, J.; Qi, X.; Min, X.-L.; Yi, W.; Liu, P.; He, Y. J. Am. Chem. Soc. 2021, 143, 10686. |
| [20] | For selected examples: (a) Bartels, B.; García-Yebra, C.; Helmchen, G. Eur. J. Org. Chem. 2003, 1097. |
| [20] | (b) Alexakis, A.; Polet, D. Org. Lett. 2004, 6, 3529. |
| [20] | (c) Tissot-Croset, K.; Polet, D.; Alexakis, A. Angew. Chem. Int. Ed. 2004, 43, 2426. |
| [20] | (d) Spiess, S.; Welter, C.; Franck, G.; Taquet, J.-P.; Helmchen, G. Angew. Chem. Int. Ed. 2008, 47, 7652. |
| [20] | (e) Spiess, S.; Raskatov, J. A.; Gnamm, C.; Brodner, K.; Helmchen, G. Chem. Eur. J. 2009, 15, 11087. |
| [20] | (f) Raskatov, J. A.; Spiess, S.; Gnamm, C.; Brodner, K.; Rominger, F.; Helmchen, G. Chem. Eur. J. 2010, 16, 6601. |
| [21] | For selected examples: (a) de Vries, A. H. M.; Meetsma, A.; Feringa, B. L. Angew. Chem. Int. Ed. 1996, 35, 2374. |
| [21] | (b) Feringa, B. L.; Pineschi, M.; Arnold, L. A.; Imbos, R.; de Vries, A. H. M. Angew. Chem. Int. Ed. 1997, 36, 2620. |
| [21] | (c) Ohmura, T.; Hartwig, J. F. J. Am. Chem. Soc. 2002, 124, 15164. |
| [21] | (d) López, F.; Ohmura, T.; Hartwig, J. F. J. Am. Chem. Soc. 2003, 125, 3426. |
| [21] | (e) Kiener, C. A.; Shu, C.; Incarvito, C.; Hartwig, J. F. J. Am. Chem. Soc. 2003, 125, 14272. |
| [21] | (f) Madrahimov, S. T.; Markovic, D.; Hartwig, J. F. J. Am. Chem. Soc. 2009, 131, 7228. |
| [22] | Leitner, A.; Shekhar, S.; Pouy, M. J.; Hartwig, J. F. J. Am. Chem. Soc. 2005, 127, 15506. |
/
| 〈 |
|
〉 |