

光氧化还原催化自由基偶联合成β-氟代-α-氨基酸衍生物
收稿日期: 2021-09-16
网络出版日期: 2021-11-02
基金资助
国家自然科学基金(21925103); 国家自然科学基金(21901062); 河南省重点研发和推广专项(科技攻关)基金(202102310005); 中国博士后科学基金(2021M690890); 河南省博士后基金(K21045Y); 河南省高等学校重点科研项目(22A150032); 河南大学的资助
Photoredox Catalytic Radical Coupling to Access β-Fluoro α-Amino Acid Derivatives
Received date: 2021-09-16
Online published: 2021-11-02
Supported by
National Natural Science Foundation of China(21925103); National Natural Science Foundation of China(21901062); Key Technologies R&D Program of Henan(202102310005); China Postdoctoral Science Foundation(2021M690890); Henan Postdoctoral Foundation(K21045Y); Key Scientific Research Projects of Henan Colleges and Universities(22A150032); Henan University
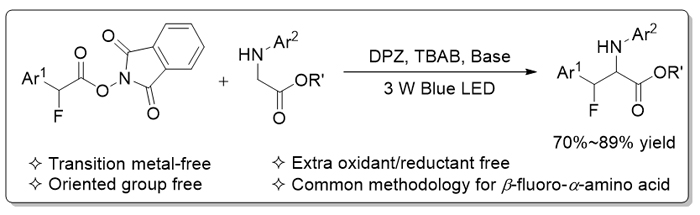
通过可见光驱动光氧化还原催化, 发展了一种新颖、便利的β-氟代-α-氨基酸衍生物的合成方法. 以非金属的二氰基吡嗪衍生物(DPZ)为光催化剂, 以易于制备的N-芳基甘氨酸酯和芳基乙酸氧化还原酯为原料, 通过单电子氧化还原分别生成酯基取代α-氨烷基自由基及α-氟代苄基自由基. 经过高反应活性自由基的交叉偶联, 高产率地得到目标产物. 该方法由于氧化还原中性反应途径而无需额外的氧化剂或还原剂, 且属于绿色、可持续的有机催化合成策略.
关键词: 光氧化还原催化; 二氰基吡嗪衍生物(DPZ); 自由基偶联; N-芳基甘氨酸酯; 芳基乙酸氧化还原酯; β-氟代-α-氨基酸
贾红绍 , 乔保坤 , 江智勇 . 光氧化还原催化自由基偶联合成β-氟代-α-氨基酸衍生物[J]. 化学学报, 2021 , 79(12) : 1477 -1480 . DOI: 10.6023/A21090432
Photoredox catalysis is a practical and efficient synthetic technique that enables previously challenging or even impossible chemical transformations proceeding effectively. As one of the most prominent contribution to green chemistry, it provides a sustainable platform to the generation of high reactive radical species under mild reaction conditions. On the other hand, β-fluoro α-amino acids are significant structural units present in enzyme inhibitors, drugs and probes. Catalytic β-C(sp3)―H fluorination of α-amino acid represents a direct synthetic approach, but the harsh reaction conditions and the limited substrate scopes lead to high difficulty for these methodologies to being generalized to industrial application. Here, we report a novel and modular protocol that is via photoredox catalytic radical coupling. By using a dicyanopyrazine-derived chromophore (DPZ) as the photoredox catalyst, two readily accessible starting substrates, that are N-aryl glycine esters and α-fluoro-aryl acetic acid-derived redox-active esters (RAEs), can undergo single-electron oxidation and reduction, respectively. The resulting ester-substituted α-amino radicals and α-fluoro benzylic radicals then experience cross coupling, a highly reactive process in radical chemistry. As a result, a series of β-fluoro-α-amino acid derivatives were obtained in high yields. In this transition metal-free catalytic system, no extra oxidant or reductant is required, representing a redox neutral platform. General procedure for the synthesis of β-fluoro-α-amino acid derivatives is: to a flame dried Schlenk tube was sequentially added N-aryl substituted glycine esters 1 (0.4 mmol), RAEs 2 (0.2 mmol), DPZ (0.004 mmol, 1.42 mg), tetra-n-butyl- ammonium bromide (0.04 mmol, 12.9 mg), sodium dihydrogen phosphate (0.40 mmol, 48 mg) and cyclopentyl methyl ester (CPME) (4 mL). Then degassed three times by freeze-pump-thaw method. The reaction mixture was stirred under an argon atmosphere at 25 ℃ irradiated by a 3 W blue LED for 48 h. After completion of the reaction, the reaction mixture was directly loaded onto a short basified silica gel column, followed by gradient elution with petroleum ether/ethyl acetate (V/V, 8/1). Removing the solvent in vacuo, afforded products.

| [1] | For selected reviews, see: (a) Yoder, N. C.; Kumar, K. Chem. Soc. Rev. 2002, 31, 335. |
| [1] | (b) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320. |
| [1] | (c) Salwiczek, M. Chem. Soc. Rev. 2012, 41, 2135. |
| [1] | (d) Tan, F.; Xiao, W. Acta Chim. Sinica 2015, 73, 85 (in Chinese). |
| [1] | ( 谭芬, 肖文精, 化学学报, 2015, 73, 85.) |
| [2] | For selected reviews, see: (a) Eriksson, T.; Bjöurkman, S.; Roth, B.; Fyge, Å.; Höuglund, P. Chirality 1995, 7, 44. |
| [2] | (b) Okarvi, S. Eur. J. Nucl. Med. 2001, 28, 929. |
| [2] | (c) Yu, W.; McConathy, J.; Williams, L.; Camp, V. M.; Malveaux, E. J.; Zhang, Z.; Olson, J. J.; Goodman, M. M. J. Med. Chem. 2009, 53, 876. |
| [2] | (d) Buer, B. C.; Marsh, E. N. G. Protein Sci. 2012, 21, 453. |
| [2] | (e) Huang, C.; McConathy, J. Curr. Top. Med. Chem. 2013, 13, 871. |
| [2] | (f) Yu, W.; McConathy, J.; Olson, J. J.; Goodman, M. M. Nucl. Med. Biol. 2015, 42, 8. |
| [2] | (g) Bouhlel, A.; Zhou, D.; Li, A.; Yuan, L.; Rich, K. M.; McConathy, J. J. Med. Chem. 2015, 58, 3817. |
| [3] | (a) Wade, T. N. J. Org. Chem. 1980, 45, 5328. |
| [3] | (b) Hart, B. P.; Coward, J. K. Tetrahedron Lett. 1993, 34, 4917. |
| [3] | (c) Kukhar, V. P. J. Fluorine Chem. 1994, 69, 199. |
| [3] | (d) Shi, G.-Q.; Zhao, Z.-Y.; Zhang, X.-B. J. Org. Chem. 1995, 60, 6608. |
| [3] | (e) Ayi, A. I.; Guedj, R.; Septe, B. J. Fluorine Chem. 1995, 73, 165. |
| [4] | (a) Zhu, R.-Y.; Tanaka, K.; Li, G.-C.; He, J.; Fu, H.-Y.; Li, S.-H.; Yu, J.-Q. J. Am. Chem. Soc. 2015, 137, 7067. |
| [4] | (b) Zhang, Q.; Yin, X.-S.; Chen, K.; Zhang, S.-Q.; Shi, B.-F. J. Am. Chem. Soc. 2015, 137, 8219. |
| [4] | (c) Bume, D. D.; Pitts, C. R.; Jokhai, R. T.; Lectka, T. Tetrahedron 2016, 72, 6031. |
| [5] | For selected reviews, see: (a) Xuan, J.; Xiao, W.-J. Angew. Chem. Int. Ed. 2012, 51, 6828. |
| [5] | (b) Chen, J.-R.; Hu, X.-Q.; Lu, L.-Q.; Xiao, W.-J. Chem. Soc. Rev. 2016, 45, 2044. |
| [5] | (c) Stephenson, C. R. J.; Yoon, T. P.; MacMillan, D. W. C. Visible Light Photocatalysis in Organic Chemistry, John Wiley & Sons, New Jersey, 2018. |
| [5] | (d) Qin, Y.; Zhu, L.; Luo, S. Chem. Rev. 2017, 117, 9433. |
| [5] | (e) Liu, Q.; Wu, L.-Z. Natl. Sci. Rev. 2017, 4, 359. |
| [5] | (f) Qiao, B.; Jiang, Z. ChemPhotoChem 2018, 2, 703. |
| [5] | (g) Chen, Y.; Lu, L.-Q.; Yu, D.-G.; Zhu, C.-J.; Xiao, W.-J. Sci. China Chem. 2019, 62, 24. |
| [5] | (h) Ren, L.; Ran, M.; He, J.; Qian, Y.; Yao, Q. Chin. J. Org. Chem. 2019, 39, 1583 (in Chinese). |
| [5] | ( 任林静, 冉茂刚, 何佳芯, 钱燕, 姚秋丽, 有机化学, 2019, 39, 1583.) |
| [6] | For selected reviews, see: (a) Yin, Y.; Zhao, X.; Qiao, B.; Jiang, Z. Org. Chem. Front. 2020, 7, 1283. |
| [6] | (b) Lv, X.; Xu, H.; Yin, Y.; Zhao, X.; Jiang, Z. Chin. J. Chem. 2020, 38, 1480. |
| [6] | (c) Srivastava, V.; Singh, P. K.; Srivastava, A.; Sinha, S.; Singh, P. P. Photochem 2021, 1, 237. |
| [7] | For selected reviews, see: (a) Li, J.; Kong, M.; Qiao, B.; Lee, R.; Zhao, X.; Jiang, Z. Nat. Commun. 2018, 9, 2445. |
| [7] | (b) Liu, Y.; Liu, X.; Li, J.; Zhao, X.; Qiao, B.; Jiang, Z. Chem. Sci. 2018, 9, 8094. |
| [7] | (c) Yin, Y.; Dai, Y.; Jia, H.; Li, J.; Bu, L.; Qiao, B.; Zhao, X.; Jiang, Z. J. Am. Chem. Soc. 2018, 140, 6083. |
| [8] | (a) Wang, C.; Guo, M.; Qi, R.; Shang, Q.; Liu, Q.; Wang, S.; Zhao, L.; Wang, R.; Xu, Z. Angew. Chem. Int. Ed. 2018, 57, 15841. |
| [8] | (b) Zhu, Z.; Xiao, L.; Xie, Z.; Le, Z. Chin. J. Org. Chem. 2019, 39, 2345 (in Chinese). |
| [8] | ( 祝志强, 肖利金, 谢宗波, 乐长高, 有机化学, 2019, 39, 2345.) |
| [9] | For selected reviews, see: (a) Cheng, W.-M.; Shang, R.; Fu, Y. ACS Catal. 2016, 7, 907. |
| [9] | (b) Fawcett, A.; Pradeilles, J.; Wang, Y.; Mutsuga, T.; Myers, E. L.; Aggarwal, V. K. Science 2017, 357, 283. |
| [9] | (c) Zhao, W.; Wurz, R. P.; Peters, J. C.; Fu, G. C. J. Am. Chem. Soc. 2017, 139, 12153. |
| [9] | (d) Mao, R.; Frey, A.; Balon, J.; Hu, X. Nat. Catal. 2018, 1, 120. |
| [10] | Liu, X.; Liu, Y.; Chai, G.; Qiao, B.; Zhao, X.; Jiang, Z. Org. Lett. 2018, 20, 6298. |
| [11] | (a) Liu, X.; Ye, X.; Bureš, F.; Liu, H.; Jiang, Z. Angew. Chem. Int. Ed. 2015, 54, 11443. |
| [11] | (b) Lin, L.; Bai, X.; Ye, X.; Zhao, X.; Tan, C.-H.; Jiang, Z. Angew. Chem. Int. Ed. 2017, 56, 13842. |
| [11] | (c) Shao, T.; Jiang, Z. Acta Chim. Sinica 2017, 75, 80 (in Chinese). |
| [11] | ( 邵天举, 江智勇, 化学学报, 2017, 75, 80.) |
| [11] | (d) Hou, M.; Lin, L.; Chai, X.; Zhao, X.; Qiao, B.; Jiang, Z. Chem. Sci. 2019, 10, 6629. |
| [11] | (e) Qiao, B.; Li, C.; Zhao, X.; Yin, Y.; Jiang, Z. Chem. Commun. 2019, 55, 7534. |
| [11] | (f) Shao, T.; Li, Y.; Ma, N.; Li, C.; Chai, G.; Zhao, X.; Qiao, B.; Jiang, Z. iScience 2019, 16, 410. |
| [11] | (g) Yin, Y.; Li, Y.; Gonçalves, T. P.; Zhan, Q.; Wang, G.; Zhao, X.; Qiao, B.; Huang, K.-W.; Jiang, Z. J. Am. Chem. Soc. 2020, 142, 19451. |
| [11] | (h) Kong, M.; Tan, Y.; Zhao, X.; Qiao, B.; Tan, C.-H.; Cao, S.; Jiang, Z. J. Am. Chem. Soc. 2021, 143, 4024. |
| [12] | (a) Oscarson, S. Protective Group Strategies, CRC Press, Taylor Francis Group, Boca Raton, 2006. |
| [12] | (b) Wuts, P. G.; Greene, T. W. Greene's Protective Groups in Organic Synthesis, John Wiley & Sons, New Jersey, 2006. |
| [13] | (a) Walling, C. Free Radicals in Solution, John Wiley & Sons, Inc., New York, 1957. |
| [13] | (b) Curran, D. P.; Porter, N. A.; Giese, B. Stereochemistry of Radical Reactions:Concepts, Guidelines and Synthetic Applications, Wiley-VCH Verlag, Weinheim, Germany, 1996. |
| [13] | (c) Roberts, B. P. Chem. Soc. Rev. 1999, 28, 25. |
| [13] | (d) Hata, S.; Sibi, M. P. Addition of Free Radicals to Carbon-Carbon Multiple Bonds, In Stereoselective Synthesis, Vol. 1, Eds.: De Vries, J. G.; Molander, G. A.; Evans, P. A., Georg Thieme Verlag, Stuttgart, Germany, 2011. |
| [13] | (e) Wille, U. Chem. Rev. 2013, 113, 813. |
| [14] | See the Supporting Information for details. |
/
| 〈 |
|
〉 |