

捕捉环加成反应中的有机亚铜中间体构筑氮杂环化合物研究进展
收稿日期: 2022-10-10
网络出版日期: 2022-11-21
基金资助
项目受国家自然科学基金(22001093); 项目受国家自然科学基金(21971087); 广东省基础与应用基础研究项目(2022A1515010200)
Recent Advances in the Construction of Nitrogen-Containing Heterocycles via Trapping Organocopper(I) Intermediates
Received date: 2022-10-10
Online published: 2022-11-21
Supported by
National Natural Science Foundation of China(22001093); National Natural Science Foundation of China(21971087); Guangdong Basic and Applied Basic Research Foundation(2022A1515010200)
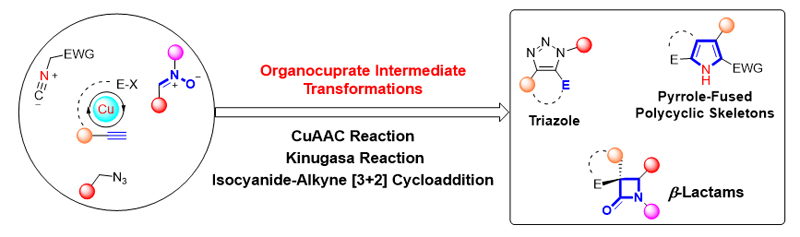
铜(I)盐催化的环加成反应, 如叠氮-炔[3+2]环加成(CuAAC)、不饱和化合物与异氰基化合物的[3+2]环加成、硝酮-炔的环加成(Kinugasa反应)是构建多类氮杂环的高效合成方法, 被广泛应用于有机合成的各个领域. 近年来, 针对几类环加成反应中产生的有机亚铜中间体的多样性转化吸引了国内外很多课题组的注意, 基于对这些环加成反应中有机亚铜中间体的捕捉, 多类串联及多组分反应得以发展, 从而成功实现了一系列多取代杂环或稠环结构的高效构建. 本综述总结了这一领域的研究进展, 按照所经历的有机亚铜中间体的类型进行分类, 包括: (1) CuAAC反应中产生的三氮唑亚铜中间体; (2) 炔烃与异氰化合物[3+2]环加成反应中产生的2H-吡咯基亚铜中间体; (3) Kinugasa反应中产生的烯醇亚铜中间体. 期望此综述能够有助于研究者了解有机亚铜中间体捕捉策略的发展、应用现状及不足之处, 进一步推动铜催化转化的发展.
邱孔茜 , 李杰 , 马浩文 , 周伟 , 蔡倩 . 捕捉环加成反应中的有机亚铜中间体构筑氮杂环化合物研究进展[J]. 化学学报, 2023 , 81(1) : 42 -63 . DOI: 10.6023/A22100419
Copper(I)-catalyzed cycloaddition reactions, such as [3+2] cycloaddition of organic azides with terminal alkynes (CuAAC), unsaturated compounds with isocyanides, and nitrones with alkynes (Kinugasa reaction), are important methods for the constructions of different types of nitrogen-containing heterocycles. Great progresses have been achieved in such transformations and widely applied in various fields of organic syntheses. In these cycloaddition reactions, similar organocopper(I) intermediates are generated in situ, and can be trapped with additional electrophiles for further tandem or one-pot transformations. The development of the strategy to trap organocopper(I) intermediates in these reactions provides practical and efficient methods for the construction of multisubstituted or fused nitrogen-containing heterocycles. The advances in this area are summarized in this review: (1) trapping organocopper(I) intermediates in CuAAC reaction; (2) trapping organocopper(I) intermediates in the [3+2] cycloaddition of isocyanides with electron-deficient alkynes; (3) trapping enolate organocopper(I) intermediates in Kinugasa reaction. The review may be helpful for researchers to better understand the development and limitations for the trapping of organocopper(I) intermediates.

| [1] | Gilman, H.; Jones, R. G.; Woods, L. A. J. Org. Chem. 1952, 17, 1630. |
| [2] | (a) Krause, N. Modern Organocopper Chemistry, Wiley-VCH, Weinheim, Germany, 2002. |
| [2] | (b) Woodward, S. Chem. Soc. Rev. 2000, 29, 393. |
| [2] | (c) Yoshikai, N.; Nakamura, E. Chem. Rev. 2012, 112, 2339. |
| [2] | (d) Tang, H.; Huang, D.; Li, Y.; Du, X.; Lian, H.; Chen, J. Acta Chim. Sinica 1994, 52, 306. (in Chinese) |
| [2] | ( 唐洪春, 黄道孝, 李玉林, 杜秀宝, 连洪寿, 陈冀胜, 化学学报 1994, 52, 306.) |
| [3] | Kharasch, M. S.; Tawney, P. O. J. Am. Chem. Soc. 1941, 63, 2308. |
| [4] | For selected reviews, see: (a) Reymond, S.; Cossy, J. Chem. Rev. 2008, 108, 5359. |
| [4] | (b) Stanley, L. M.; Sibi, M. P. Chem. Rev. 2008, 108, 2887. |
| [4] | (c) Hein, J. E.; Fokin, V. V. Chem. Soc. Rev. 2010, 39, 1302. |
| [4] | (d) Khangaro, R. K.; Kaliappan, K. P. Eur. J. Org. Chem. 2013, 7664. |
| [4] | (e) Hashimoto, T.; Maruoka, K. Chem. Rev. 2015, 115, 5366. |
| [4] | (f) Haldón, E.; Nicasio, M. C.; Pérez, P. J. Org. Biomol. Chem. 2015, 13, 9528. |
| [5] | (a) Torn?e, C. W.; Christensen, C.; Meldal, M. J. Org. Chem. 2002, 67, 3057. |
| [5] | (b) Rostovtsev, V. V.; Green, L. G.; Fokin, V. V.; Sharpless, K. B. Angew. Chem., Int. Ed. 2002, 41, 2596. |
| [5] | (c) Dong, J.; Xu, L. Chin. J. Chem. 2020, 38, 414. |
| [6] | (a) Huisgen, R. Angew. Chem., Int. Ed. 1963, 2, 565. |
| [6] | (b) Huisgen, R. Angew. Chem., Int. Ed. 1963, 2, 633. |
| [6] | (c) Huisgen, R. Pure Appl. Chem. 1989, 61, 613. |
| [7] | For selected reviews, see: (a) Meldal, M.; Torn?e, C. W. Chem. Rev. 2008, 108, 2952. |
| [7] | (b) Tiwari, V. K.; Mishra, B. B.; Mishra, K. B.; Mishra, N.; Singh, A. S.; Chen, X. Chem. Rev. 2016, 116, 3086. |
| [7] | (c) Wang, X.; Huang, B.; Liu, X.; Zhan, P. Drug Discov. Today 2016, 21, 118. |
| [7] | (d) D?hler, D.; Michael, P.; Binder, W. H. Acc. Chem. Res. 2017, 50, 2610. |
| [7] | (e) Meldal, M.; Diness, F. Trends Chem. 2020, 2, 569. |
| [8] | Nolte, C.; Mayer, P.; Straub, B. F. Angew. Chem., Int. Ed. 2007, 46, 2101. |
| [9] | Wu, Y.-M.; Deng, J.; Li, Y.; Chen, Q.-Y. Synthesis 2005, 8, 1314. |
| [10] | Cassidy, M. P.; Raushel, J.; Fokin, V. V. Angew. Chem., Int. Ed. 2006, 45, 3154. |
| [11] | Zhang, X.; Hsung, R. P.; Li, H. Chem. Commun. 2007, 2420. |
| [12] | Li, L.; Zhang, G.; Zhu, A.; Zhang, L. J. Org. Chem. 2008, 73, 3630. |
| [13] | Li, L.; Li, R.; Zhu, A.; Zhang, G.; Zhang, L. Synlett 2011, 874. |
| [14] | Malnuit, V.; Duca, M.; Manout, A.; Bougrin, K.; Benhida, R. Synlett 2009, 2123. |
| [15] | (a) Wang, B.; Ahmed, M. N.; Zhang, J.; Chen, W.; Wang, X.; Hu, Y. Tetrahedron Lett. 2013, 54, 6097. |
| [15] | (b) Wang, B.; Zhang, J.; Wang, X.; Liu, N.; Chen, W.; Hu, Y. J. Org. Chem. 2013, 78, 10519. |
| [15] | (c) Wang, B.; Liu, N.; Shao, C.; Zhang, Q.; Wang, X.; Hu, Y. Adv. Synth. Catal. 2013, 355, 2564. |
| [15] | (d) Wang, B.; Liu, N.; Chen, W.; Huang, D.; Wang, X.; Hu, Y. Adv. Synth. Catal. 2015, 357, 401. |
| [16] | Yan, R.; Sander, K.; Galante, E.; Rajkumar, V.; Badar, A.; Robson, M.; El-Emir, E.; Lythgoe, M. F.; Pedley, R. B.; ?rstad, E. J. Am. Chem. Soc. 2013, 135, 703. |
| [17] | Chen, Z.; Zhu, J.; Xie, H.; Li, S.; Wu, Y.; Gong, Y. Adv. Synth. Catal. 2010, 352, 1296. |
| [18] | Cai, Q.; Zhou, W. Chin. J. Chem. 2020, 38, 879. |
| [19] | Yan, J.; Zhou, F.; Qin, D.; Cai, T.; Ding, K.; Cai, Q. Org. Lett. 2012, 14, 1262. |
| [20] | Hooyberghs, G.; De Coster, H.; Vachhani, D. D.; Ermolat’ev, D. S.; Van der Eycken, E. V. Tetrahedron 2013, 69, 4331. |
| [21] | Vachhani, D. D.; Kumar, A.; Modha, S. G.; Sharma, S. K.; Parmar, V. S.; Van der Eycken, E. V. Eur. J. Org. Chem. 2013, 1223. |
| [22] | Reddy, A. S.; Reddy, M. N.; Swamy, K. C. K. RSC Adv. 2014, 4, 28359. |
| [23] | An, Y.; He, H.; Liu, T.; Zhang, Y.; Lu, X.; Cai, Q. Synthesis 2017, 49, 3863. |
| [24] | Xiao, G.; Wu, K.; Zhou, W.; Cai, Q. Adv. Synth. Catal. 2021, 363, 4988. |
| [25] | Cai, Q.; Yan, J.; Ding, K. Org. Lett. 2012, 14, 3332. |
| [26] | Ouyang, Y.; Si, H.; Zhu, C.; Zhong, L.; Ma, H.; Li, Z.; Xiong, H.; Liu, T.; Liu, Z.; Zhang, Z.; Zhang, Z.; Cai, Q. J. Med. Chem. 2022, 65, 7833. |
| [27] | Bag, S. S.; Das, S. K.; Gogoi, H. Tetrahedron, 2018, 74, 2218. |
| [28] | Reddy, M. N.; Swamy, K. C. K. Eur. J. Org. Chem. 2012, 2013. |
| [29] | Wei, F.; Wang, W.; Ma, Y.; Tung, C.-H.; Xu, Z. Chem. Commun. 2016, 52, 14188. |
| [30] | Ackermann, L.; Potukuchi, H. K.; Landsberg, D.; Vicente, R. Org. Lett. 2008, 10, 3081. |
| [31] | Qian, W.; Wang, H.; Allen, J. Angew. Chem., Int. Ed. 2013, 52, 10992. |
| [32] | Pericherla, K.; Jha, A.; Khungar, B.; Kumar, A. Org. Lett. 2013, 15, 4304. |
| [33] | Liu, Z.; Zhu, D.; Luo, B.; Zhang, N.; Liu, Q.; Hu, Y.; Pi, R.; Huang, P.; Wen, S. Org. Lett. 2014, 16, 5600. |
| [34] | Lautens, M.; Larin, E. M. Angew. Chem., Int. Ed. 2019, 58, 13438. |
| [35] | Wei, F.; Li, H.; Song, C.; Ma, Y.; Zhou, L.; Tung, C.-H.; Xu, Z. Org. Lett. 2015, 17, 2860. |
| [36] | (a) Zhang, Z.; Zhou, Q.; Ye, F.; Xia, Y.; Wu, G.; Hossain, M. L.; Zhang, Y.; Wang, J. Adv. Synth. Catal. 2015, 357, 2277. |
| [36] | (b) Zhang, Z.; Zhou, Q.; Yu, W.; Li, T.; Zhang, Y.; Wang, J. Chin. J. Chem. 2017, 35, 387. |
| [37] | Chen, F.-J.; Mamidipalli, P.; Sabbasani, V. R.; Liu, H.; Xia, Y.; Lee, D. Org. Chem. Front. 2021, 8, 6095. |
| [38] | Wang, W.; Wei, F.; Ma, Y.; Tung, C.-H.; Xu, Z. Org. Lett. 2016, 18, 4158. |
| [39] | (a) Zhou, W.; Zhang, M.; Li, H.; Chen, W. Org. Lett. 2017, 19, 10. |
| [39] | (b) Wu, F.; Zhou, W.; Chen, K.; Chen, W.; Liu, M.; Wu, H. Adv. Synth. Catal. 2018, 360, 2435. |
| [40] | Cheung, K. P. S.; Tsui, G. C. Org. Lett. 2017, 19, 2881. |
| [41] | Wu, W.; Wang, J.; Wang, Y.; Huang, Y.; Tan, Y.; Weng, Z. Angew. Chem., Int. Ed. 2017, 56, 10476. |
| [42] | Wang, W.; Peng, X.; Wei, F.; Tung, C.-H.; Xu, Z. Angew. Chem., Int. Ed. 2016, 55, 649. |
| [43] | Wang, W.; Huang, S.; Yan, S.; Sun, X.; Tung, C.-H.; Xu, Z. Chin. J. Chem. 2020, 38, 445. |
| [44] | Reddy, R. J.; Waheed, M.; Kumari, A. H.; Krishna, G. R. Adv. Synth. Catal. 2022, 364, 319. |
| [45] | Wei, F.; Zhou, T.; Ma, Y.; Tung, C.-H.; Xu, Z. Org. Lett. 2017, 19, 2098. |
| [46] | Yang, D.; Fu, N.; Liu, Z.; Li, Y.; Chen, B. Synlett 2007, 278. |
| [47] | Alonso, F.; Moglie, Y.; Radivoy, G.; Yus, M. Synlett 2012, 23, 2179. |
| [48] | Li, L.; Hao, G.; Zhu, A.; Fan, X.; Zhang, G.; Zhang, L. Chem. Eur. J. 2013, 19, 14403. |
| [49] | (a) Li, L.; Fan, X.; Zhang, Y.; Zhu, A.; Zhang, G. Tetrahedron 2013, 69, 9939. |
| [49] | (b) Li, L.-J.; Zhang, Y.-Q.; Zhang, Y.; Zhu, A.-L.; Zhang, G.-S. Chin. Chem. Lett. 2014, 25, 1161. |
| [50] | Selvaraju, M.; Sun, C.-M. Adv. Synth. Catal. 2014, 356, 1329. |
| [51] | (a) Peringer, F.; do Nascimento, J. E. R.; Abib, P. B.; Barcellos, T.; Van der Eycken, E. V.; Perin, G.; Jacob, R. G. Alves, D. Eur. J. Org. Chem. 2017, 2579. |
| [51] | (b) Aquino, T. B.; do Nascimento, J. E. R.; Dias, í. F. C.; de Oliveira, D. H.; Barcellos, T.; Lenard?o, E. J.; Perin, G.; Alves, D.; Jacob, R. J. Tetrahedron Lett. 2018, 59, 1080. |
| [52] | Xu, J.; Song, Q. Org. Chem. Front. 2017, 4, 938. |
| [53] | Yu, X.; Xu, J.; Zhou, Y.; Song, Q. Org. Chem. Front. 2018, 5, 2463. |
| [54] | Wang, X.-X.; Xin, Y.; Li, Y.; Xia, W.-J.; Zhou, B.; Ye, R.-R.; Li, Y.-M. J. Org. Chem. 2020, 85, 3576 |
| [55] | (a) Navarro, Y.; López, J. G.; Iglesias, M. J.; Ortiz, F. L. Org. Lett. 2021, 23, 334. |
| [55] | (b) Navarro, Y.; Jiménez, I. H.; Iglesias, M. J.; Ortiz, F. L. Synthesis 2022, 54, 199. |
| [56] | (a) Kamijo, S.; Kanazawa, C.; Yamamoto, Y. J. Am. Chem. Soc. 2005, 127, 9260. |
| [56] | (b) Larionov, O. V.; de Meijere, A. Angew. Chem., Int. Ed. 2005, 44, 5664. |
| [57] | Cai, Q.; Zhou, F.; Xu, T.; Fu, L.; Ding, K. Org. Lett. 2011, 13, 340. |
| [58] | Zhou, F.; Fu, L.; Wei, J.; Ding, K.; Cai, Q. Synthesis 2011, 3037. |
| [59] | Zhou, F.; Liu, J. Ding, K.; Liu, J.; Cai, Q. J. Org. Chem. 2011, 76, 5346. |
| [60] | Zheng, D.; Liu, T.; Liu, X.; Fan, X.; Wu, J. J. Org. Chem. 2016, 81, 9428. |
| [61] | Ouyang, Y.; Wu, K.; Zhou, W.; Cai, Q. Org. Chem. Front. 2021, 8, 2456. |
| [62] | Kinugasa, M.; Hashimoto, S. J. Chem. Soc. Chem. Commun. 1972, 466. |
| [63] | (a) Santoro, S.; Himo, F. J. Org. Chem. 2021, 86, 10665. |
| [63] | (b) Malig, T. C.; Yu, D.; Hein, J. E. J. Am. Chem. Soc. 2018, 140, 9167. |
| [63] | (c) Santoro, S.; Liao, R.-Z.; Marcelli, T.; Hammar, P.; Himo, F. J. Org. Chem. 2015, 80, 2649. |
| [64] | Shintani, R.; Fu, G. C. Angew. Chem., Int. Ed. 2003, 42, 4082. |
| [65] | Shu, T.; Zhao, L.; Li, S.; Chen, X.-Y.; von Essen, C.; Rissanen, K.; Enders, D. Angew. Chem., Int. Ed. 2018, 57, 10985. |
| [66] | Qi, J.; Wei, F.; Huang, S.; Tung, C.-H.; Xu, Z. Angew. Chem., Int. Ed. 2021, 60, 4561. |
| [67] | (a) Qi, J.; Wei, F.; Tung, C.-H.; Xu, Z. Angew. Chem., Int. Ed. 2021, 60, 13814. |
| [67] | (b) Qi, J.; Tung, C.-H.; Xu, Z. Trends Chem. 2021, 3, 984. |
| [68] | Zhong, X.; Huang, M.; Xiong, H.; Liang, Y.; Zhou, W.; Cai, Q. Angew. Chem., Int. Ed. 2022, DOI: 10.1002/anie.202208323. |
/
| 〈 |
|
〉 |