

硫酯参与的有机催化不对称反应研究进展
收稿日期: 2022-10-10
网络出版日期: 2022-12-06
基金资助
项目受辽宁省教育厅科学研究项目(LQ2020025); 南宁市科学研究与技术开发计划项目(20201043)
Advances in Organocatalytic Asymmetric Reactions Involving Thioesters
Received date: 2022-10-10
Online published: 2022-12-06
Supported by
Science and Technology Project of Liaoning Education Department(LQ2020025); Nanning Scientific Research and Technology Development Program(20201043)
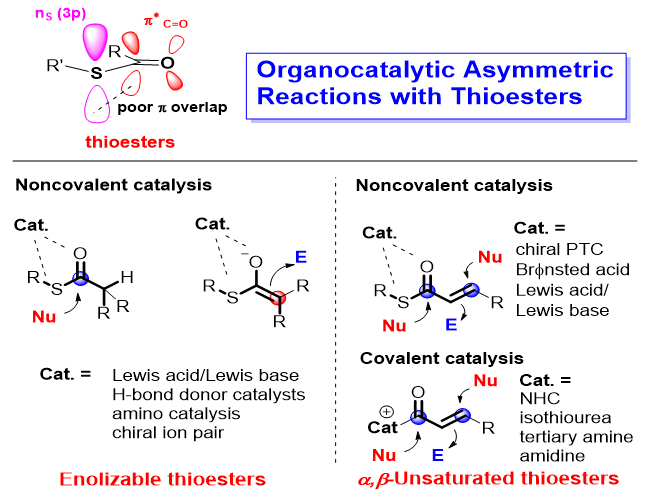
硫酯在生物合成和有机合成中扮演着十分重要的角色. 硫酯的羰基C(2p)轨道和S(3p)轨道重叠度较小, 其α质子具有较强的酸性, 能在温和的条件下烯醇化并进行亲核反应. 同时, 硫酯还是高效的酰基化试剂, 可用于酯键和酰胺键的构建. 有机小分子催化是不对称催化的重要研究领域. 近十几年来有机催化硫酯底物的不对称反应取得了大量重要成果, 极大拓宽了有机催化酯类底物的反应类型, 并实现了一些其氧酯类似物无法实现的反应. 本综述按照硫酯底物的类型(可烯醇化硫酯和α,β-不饱和硫酯)、有机催化剂类型和反应类型对硫酯底物参与的有机催化不对称反应进行梳理和总结, 同时对代表性反应的机理以及该领域的未来发展进行了简单阐述.
王晓晨 , 季泽尧 , 刘健 , 王炳福 , 金辉 , 张立新 . 硫酯参与的有机催化不对称反应研究进展[J]. 化学学报, 2023 , 81(1) : 64 -83 . DOI: 10.6023/A22100422
Thioesters play a very important role in biosynthesis and organic synthesis. Due to smaller orbital overlap of the C(2p) and S(3p) orbitals, the α-proton acidity of thioesters is higher than that of the related oxoesters, making thioesters useful enolate precursors in nature as well as in the laboratory. Meanwhile, thioesters are also efficient acylation reagents which can be used for the construction of ester bond and amide bond. Organocatalysis, a biomimetic catalysis usually with metal- free small organic molecules, is an emerging research field that has been booming since the beginning of the 21st century. In the past decade, many important achievements have been made in the organocatalytic asymmetric reactions involving thioester substrates, which have greatly broadened the reaction types of organocatalytic reactions with ester substrates and realized some reactions that cannot be achieved by using their oxoester analogues. The advances in organocatalytic asymmetric reactions involving thioesters are summarized in this review. According to the types of thioester substrates, these advances are classified to two types. One type is the organocatalytic asymmetric reactions with enolizable thioesters such as trifluoroethyl thioesters, malonic acid half-thioesters (MAHTs), monothiomalonates (MTMs) and dithiomalonates (DTMs). For these reactions, noncovalent interactions between catalysts and thioesters, including hydrogen bonding and ion pair interaction, have been used to promote the reaction and to achieve the high enantioselectivity. Another type is the catalytic asymmetric reactions with α,β-unsaturated thioesters. For the reaction of this type, various chiral organocatalysts, including chiral amines, ureas, NHC (N-heterocyclic carbene), isothiourea, amidine and others, not only activate the thioester substrates, but also control the enantioselectivity well through covalent and non-covalent bonds. Meanwhile, the mechanism of representative transformations will be briefly introduced and at last, the perspective in this area will be given.

Key words: thioesters; organocatalysis; asymmetric reaction; biomimetic synthesis
| [1] | (a) Ding, K. L.; Fan, Q. H. Asymmetric Catalysis: New Concepts and Methods, Chemical Industry Press, Beijing, 2009. (in Chinese) |
| [1] | ( 丁奎岭, 范青华, 不对称催化新概念与新方法, 化学工业出版社, 北京, 2009.) |
| [1] | (b) Ding, K. L. Acta Chim. Sinica 2014, 72, 755. (in Chinese) |
| [1] | ( 丁奎岭, 化学学报 2014, 72, 755.) |
| [1] | (c) Dalko, P. I. Comprehensive Enantioselective Organocatalysis: Catalysts, Reactions, and Applications, Wiley-VCH Verlag GmbH, Weinheim, 2013. |
| [2] | Saha, D.; Kharbanda, A.; Yan, W.; Lakkaniga, N. R.; Frett, B.; Li, H.-y. J. Med. Chem. 2020, 63, 441. |
| [3] | (a) Mulholland, A. J.; Lyne, P. D.; Karplus, M. J. Am. Chem. Soc. 2000, 122, 534. |
| [3] | (b) Usher, K. C.; Remington, S. J.; Martin, D. P.; Drueckhammer, D. G. Biochemistry 1994, 33, 7753. |
| [3] | (c) Wiegand, G.; Remington, S. J. Ann. Rev. Biophys. Chem. 1986, 15, 97. |
| [4] | (a) Hill, A. M. Nat. Prod. Rep. 2006, 23, 256. |
| [4] | (b) Staunton, J.; Weissman, K. J. Nat. Prod. Rep. 2001, 18, 380. |
| [5] | (a) Masamune, S.; Kamata, S.; Schilling, W. J. Am. Chem. Soc. 1975, 97, 3515. |
| [5] | (b) Masamune, S.; Hayase, Y.; Schilling, W.; Chan, W. K.; Bates, G. S. J. Am. Chem. Soc. 1977, 99, 6756. |
| [6] | Yang, X.; Ma, Y.; Di, H.; Wang, X.; Jin, H.; Ryu, D. H.; Zhang, L. Adv. Synth. Catal. 2021, 363, 3201. |
| [7] | (a) Tokuyama, H.; Yokoshima, S.; Yamashita, T.; Fukuyama, T. Tetrahedron Lett. 1998, 39, 3189. |
| [7] | (b) Miyazaki, T.; Han-ya, Y.; Tokuyama, H.; Fukuyama, T. Synlett 2004, 477. |
| [7] | (c) Fukuyama, T.; Tokuyama, H. Aldrichimica Acta 2004, 37, 87. |
| [7] | (d) Mori, Y.; Seki, M. Adv. Synth. Catal. 2007, 349, 2027. |
| [7] | (e) Cherney, A. H.; Reisman, S. E. Tetrahedron 2014, 70, 3259. |
| [8] | Um, P.-J.; Drueckhammer, D. G. J. Am. Chem. Soc. 1998, 120, 5607. |
| [9] | (a) Hirschbeck, V.; Gehrtz, P. H.; Fleischer, I. Chem. Eur. J. 2018, 24, 7092. |
| [9] | (b) Ye, Q.; Cao, W.-G.; Gao, J.-S. Chin. J. Org. Chem. 2002, 21, 697. (in Chinese) |
| [9] | ( 叶青, 曹卫国, 高金森, 有机化学, 2002, 21, 697.) |
| [10] | (a) Magdziak, D.; Lalic, G.; Lee, M. H.; Fortner, K. C.; Aloise, A. D.; Shair, M. D. J. Am. Chem. Soc. 2005, 127, 7284. |
| [10] | (b) Orlandi, S.; Benaglia, M.; Cozzi, F. Tetrahedron Lett. 2004, 45, 1747. |
| [10] | (c) Sawa, M.; Miyazaki, S.; Yonesaki, R.; Morimoto, H.; Ohshima, T. Org. Lett. 2018, 20, 5393. |
| [10] | (d) Furutachi, M.; Mouri, S.; Matsunaga, S.; Shibasaki, M. Chem. Asian J. 2010, 5, 2351. |
| [11] | Walker, M. C.; Thuronyi, B. W.; Charkoudian, L. K.; Lowry, B.; Khosla, C.; Chang, M. C. Y. Science 2013, 341, 1089. |
| [12] | (a) Xiang, S.-H.; Tan, B. Nat. Commun. 2020, 11, 3786. |
| [12] | (b) Zhang, M.-M.; Luo, Y.-Y.; Lu, L.-Q.; Xiao, W.-J. Acta Chim. Sinica 2018, 76, 838. (in Chinese) |
| [12] | ( 张毛毛, 骆元元, 陆良秋, 肖文精, 化学学报 2018, 76, 838.) |
| [12] | (c) Di, H.; Liu, Y.; Ma, Y.; Yang, X.; Jin, H.; Zhang, L. Chin. J. Org. Chem. 2021, 41, 2228. (in Chinese) |
| [12] | ( 底慧明, 刘云婷, 马艳榕, 杨鑫悦, 金辉, 张立新, 有机化学, 2021, 41, 2228.) |
| [12] | (d) Ren, H.; Ma, M.; Huang, Y. Chin. J. Org. Chem. 2022, 42, 3129. (in Chinese) |
| [12] | 任红霞, 马萌萌, 黄有, 有机化学, 2022, 42, 3129.). |
| [12] | (e) Chen, X.; Wang, H.; Jin, Z.; Chi, Y. R. Chin. J. Chem. 2020, 38, 1167. |
| [13] | Bordwell, F. G.; Fried, H. E. J. Org. Chem. 1991, 56, 4218. |
| [14] | Um, P.-J.; Drueckhammer, D. G. J. Am. Chem. Soc. 1998, 120, 5605. |
| [15] | Alonso, D. A.; Kitagaki, S.; Utsumi, N.; Barbas III, C. F. Angew. Chem., Int. Ed. 2008, 47, 4588. |
| [16] | Hayashi, Y.; Yamada, T.; Sato, M.; Watanabe, S.; Kwon, E.; Iwasaki, K.; Umemiya, S. Org. Lett. 2019, 21, 5183. |
| [17] | Kohler, M. C.; Yost, J. M.; Garnsey, M. R.; Coltart, D. M. Org. Lett. 2010, 12, 3376. |
| [18] | Clayden, J.; Greeves, N.; Warren, S. Organic Chemistry, 2nd ed., Oxford University Press, New York, 2012, pp. 628-630. |
| [19] | Kobuke, Y.; Yoshida, J.-i. Tetrahedron Lett. 1978, 19, 367. |
| [20] | (a) Magdziak, D.; Lalic, G.; Lee, H. M.; Fortner, K. C.; Aloise, A. D.; Shair, M. D. J. Am. Chem. Soc. 2005, 127, 7284. |
| [20] | (b) Fortner, K. C.; Shair, M. D. J. Am. Chem. Soc. 2007, 129, 1032. |
| [20] | (c) Lalic, G.; Aloise, A. D.; Shair, M. D. J. Am. Chem. Soc. 2003, 125, 2852. |
| [20] | (d) Orlandi, S.; Benaglia, M.; Cozzi, F. Tetrahedron Lett. 2004, 45, 1747. |
| [21] | Bae, H. Y.; Sim, J. H.; Lee, J.-W.; List, B.; Song, C. E. Angew. Chem., Int. Ed. 2013, 52, 12143. |
| [22] | Wang, Y.; Huang, G.; Hu, S.; Jin, K.; Wu, Y.; Chen, F. Tetrahedron Lett. 2017, 73, 5055. |
| [23] | (a) Müller, K.; Faeh, C.; Diederich, F. Science 2007, 317, 1881. |
| [23] | (b) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 32. |
| [23] | (c) O'Hagan, D. Chem. Soc. Rev. 2008, 37, 308. |
| [24] | Saadi, J.; Akakura, M.; Yamamoto, H. J. Am. Chem. Soc. 2011, 133, 14248. |
| [25] | Saadi, J.; Wennemers, H. Nat. Chem. 2016, 8, 276. |
| [26] | Zetschok, D.; Heieck, L.; Wennemers, H. Org. Lett. 2021, 23, 1753. |
| [27] | (a) Bao, Y.; Kumagai, N.; Shibasaki, M. Chem. Sci. 2015, 6, 6124. |
| [27] | (b) Misaki, T.; Takimoto, G.; Sugimura, T. J. Am. Chem. Soc. 2010, 132, 6286. |
| [27] | (c) Yamaguchi, A.; Matsunaga, S.; Shibasaki, M. J. Am. Chem. Soc. 2009, 131, 10842. |
| [27] | (d) Saito, S.; Kobayashi, S. J. Am. Chem. Soc. 2006, 128, 8704. |
| [27] | (e) Suto, Y.; Tsuji, R.; Kanai, M.; Shibasaki, M. Org. Lett. 2005, 7, 3757. |
| [28] | Park, J. H.; Sim, J. H.; Song, C. E. Org. Lett. 2019, 21, 4567. |
| [29] | Ricci, A.; Pettersen, D.; Bernardi, L.; Fini, F.; Fochi, F.; Herrera, R. P.; Sgarzania, V. Adv. Synth. Catal. 2007, 349, 1037. |
| [30] | Pan, Y. H.; Kee, C. W.; Jiang, Z. Y.; Ma, T.; Zhao, Y. J.; Yang, Y. Y.; Xue, H. S.; Tan, C. H. Chem. Eur. J. 2011, 17, 8363. |
| [31] | Hara, N.; Nakamura, S.; Sano, M. Chem. Eur. J. 2012, 18, 9276. |
| [32] | (a) Takashina, N.; Price, C. C. J. Am. Chem. Soc. 1962, 84, 489. |
| [32] | (b) Rauhut, M. M.; Currier, H. A.; Semsel, A. M.; Wystrach, V. P. J. Org. Chem. 1961, 26, 5138. |
| [32] | (c) Morita, K.-i.; Suzuki, Z.; Hirose, H. Bull. Chem. Soc. Jpn. 1968, 41, 2815. |
| [33] | (a) Lu, X.; Zhang, C.; Xu, Z. Acc. Chem. Res. 2001, 34, 535. |
| [33] | (b) Methot, J. L.; Roush, W. R. Adv. Synth. Catal. 2004, 346, 1035. |
| [33] | (c) Lu, X.; Du, Y.; Lu, C. Pure Appl. Chem. 2005, 77, 1985. |
| [33] | (d) Ye, L.-W.; Zhou, J.; Tang, Y. Chem. Soc. Rev. 2008, 37, 1140. |
| [33] | (e) Cowen, B. J.; Miller, S. J. Chem. Soc. Rev. 2009, 38, 3102. |
| [33] | (f) Wei, Y.; Shi, M. Acc. Chem. Res. 2010, 43, 1005. |
| [33] | (g) Marinetti, A.; Voituriez, A. Synlett 2010, 2010, 174. |
| [33] | (h) Lu, Y.; Wang, S.-X.; Han, X.; Zhong, F.; Wang, Y. Synlett 2011, 2011, 2766. |
| [33] | (i) Zhao, Q.-Y.; Lian, Z.; Wei, Y.; Shi, M. Chem. Commun. 2012, 48, 1724. |
| [33] | (j) Fan, Y. C.; Kwon, O. Chem. Commun. 2013, 49, 11588. |
| [33] | (k) Wang, Z.; Xu, X.; Kwon, O. Chem. Soc. Rev. 2014, 43, 2927. |
| [33] | (l) Xiao, Y.; Sun, Z.; Guo, H.; Kwon, O. J. Org. Chem. 2014, 10, 2089. |
| [33] | (m) Wang, T.; Han, X.; Zhong, F.; Yao, W.; Lu, Y. Acc. Chem. Res. 2016, 49, 1369. |
| [33] | (n) Li, W.; Zhang, J. Chem. Soc. Rev. 2016, 45, 1657. |
| [33] | (o) Ni, H.; Chan, W.-L.; Lu, Y. Chem. Rev. 2018, 118, 9344. |
| [33] | (p) Guo, H.; Fan, Y. C.; Sun, Z.; Wu, Y.; Kwon, O. Chem. Rev. 2018, 118, 10049. |
| [34] | (a) White, D. A.; Baizer, M. M. Tetrahedron Lett. 1973, 14, 3597. |
| [34] | (b) Trost, B. M.; Li, C.-J. J. Am. Chem. Soc. 1994, 116, 10819. |
| [34] | (c) Trost, B. M.; Li, C.-J. J. Am. Chem. Soc. 1994, 116, 3167. |
| [34] | (d) Trost, B. M.; Dake, G. R. J. Org. Chem. 1997, 62, 5670. |
| [34] | (e) Zhang, C.; Lu, X. Synlett 1995, 1995, 645. |
| [34] | (f) Chung, Y. K.; Fu, G. C. Angew. Chem., Int. Ed. 2009, 48, 2225. |
| [34] | (g) Smith, S. W.; Fu, G. C. J. Am. Chem. Soc. 2009, 131, 14231. |
| [34] | (h) Sun, J.; Fu, G. C. J. Am. Chem. Soc. 2010, 132, 4568. |
| [34] | (i) Lundgren, R. J.; Wilsily, A.; Marion, N.; Ma, C.; Chung, Y. K.; Fu, G. C. Angew. Chem., Int. Ed. 2013, 52, 2525. |
| [34] | (j) Chen, Z.; Zhu, G.; Jiang, Q.; Xiao, D.; Cao, P.; Zhang, X. J. Org. Chem. 1998, 63, 5631. |
| [35] | (a) Zhong, F.; Dou, X.; Han, X.; Yao, W.; Zhu, Q.; Meng, Y.; Lu, Y. Angew. Chem., Int. Ed. 2013, 52, 943. |
| [35] | (b) Wang, T.; Yao, W.; Zhong, F.; Pang, G. H.; Lu, Y. Angew. Chem., Int. Ed. 2014, 126, 3008. |
| [35] | (c) Wang, T.; Yu, Z.; Hoon, D. L.; Huang, K.-W.; Lan, Y.; Lu, Y. Chem. Sci. 2015, 6, 4912. |
| [35] | (d) Wang, T.; Hoon, D. L.; Lu, Y. Chem. Commun. 2015, 51, 10186. |
| [35] | (e) Huang, G.; Ren, X.; Jiang, C.; Wu, J.-H.; Gao, G.; Wang, T. Org. Chem. Front. 2019, 6, 2872. |
| [36] | Wang, X.; Fang, F.; Zhao, C.; Tian, S.-K. Tetrahedron Lett. 2008, 49, 6442. |
| [37] | (a) Wang, H.-Y.; Zhang, K.; Zheng, C.-W.; Chai, Z.; Cao, D.-D.; Zhang, J.-X.; Zhao, G. Angew. Chem., Int. Ed. 2015, 54, 1775. |
| [37] | (b) Lou, Y.-P.; Zheng, C.-W.; Pan, R.-M.; Jin, Q.-W.; Zhao, G.; Li, Z. Org. Lett. 2015, 17, 688. |
| [37] | (c) Xu, L.; Wang, H.; Zheng, C.; Zhao, G. Adv. Synth. Catal. 2017, 359, 2942. |
| [37] | (d) Pan, R.; Zhang, J.; Zheng, C.; Wang, H.; Cao, D.; Cao, W.; Zhao, G. Tetrahedron 2017, 73, 2349. |
| [37] | (e) Ji, X.; Cao, W.-G.; Zhao, G. Tetrahedron 2017, 73, 5983. |
| [37] | (f) Jin, Q.; Zheng, C.; Zhao, G.; Zou, G. Tetrahedron 2018, 74, 4134. |
| [37] | (g) Wang, H.-Y.; Zheng, C.-W.; Chai, Z.; Zhang, J.-X.; Zhao, G. Nat. Commun. 2016, 7, 12720. |
| [38] | Zhang, H.; Jiang, C.; Tan, J.-P.; Hu, H.-L.; Chen, Y.; Ren, X.; Zhang, H.-S.; Wang, T. ACS Catal. 2020, 10, 5698. |
| [39] | (a) Fleming, F. F. Nat. Prod. Rep. 1999, 16, 597. |
| [39] | (b) Miller, J. S.; Manson, J. L. Acc. Chem. Res. 2001, 34, 563. |
| [39] | (c) Lindquist, B. A.; Furse, K. E.; Corcelli, S. A. Phys. Chem. 2009, 11, 8119. |
| [40] | (a) Laine, D.; Palovich, M.; McCleland, B.; Petitjean, E.; Delhom, I.; Xie, H.; Deng, J.; Lin, G.; Davis, R.; Jolit, A.; Nevins, N.; Zhao, B.; Villa, J.; Schneck, J.; McDevitt, P.; Midgett, R.; Kmett, C.; Umbrecht, S.; Peck, B.; Davis, A.-B.; Bettoun, D. ACS Med. Chem. Lett. 2011, 2, 142. |
| [40] | (b) Raja, V.-P.-A.; Perumal, S.; Yogeeswari, P.; Sriram, D. Bioorg. Med. Chem. Lett. 2011, 21, 3881. |
| [40] | (c) Casimiro-Garcia, A.; Trujillo, J.-I.; Vajdos, F.; Juba, B.; Banker, M.-E.; Aulabaugh, A.; Balbo, P.; Bauman, J.; Chrencik, J.; Coe, J.-W.; Czerwinski, R.; Dowty, M.; Knafels, J.-D.; Kwon, S.; Leung, L.; Liang, S.; Robinson, R.-P.; Telliez, J.-B.; Unwalla, R.; Yang, X.; Thorarensen, A. J. Med. Chem. 2018, 61, 10665. |
| [41] | Oyamada, Y.; Inaba, K.; Sasamori, T.; Nakamura, S. Chem. Commun. 2022, 58, 2172. |
| [42] | Lubkoll, J.; Wennemers, H. Angew. Chem., Int. Ed. 2007, 46, 6841. |
| [43] | Bae, H. Y.; Some, S.; Lee, J. H.; Kim, J.-Y.; Song, M. J.; Lee, S.; Zhang, Y. J.; Song, C. E. Adv. Synth. Catal. 2011, 353, 3196. |
| [44] | (a) Chiu, P.; Leung, L. T.; Ko, B. C. B. Nat. Prod. Rep. 2010, 27, 1066. |
| [44] | (b) Florence, G. J.; Gardnerb, N. M.; Paterson, I. Nat. Prod. Rep. 2008, 25, 342. |
| [44] | (c) Boucard, V.; Broustal, G.; Campagne, J. M. Eur. J. Org. Chem. 2007, 225. |
| [44] | (d) Mondon, M.; Gesson, J.-P. Curr. Org. Synth. 2006, 3, 41. |
| [45] | Ren, Q.; Sun, S.; Huang, J.; Li, W.; Wu, M.; Guo, H.; Wang, J.: Chem. Commun. 2014, 50, 6137. |
| [46] | Clerici, P.; Wennemers, H. Org. Biomol. Chem. 2012, 10, 110. |
| [47] | Kolarovic, A.; K?slin, A.; Wennemers, H. Org. Lett. 2014, 16, 4236. |
| [48] | Ling, T.; Rivas, F. Tetrahedron 2016, 72, 6729. |
| [49] | (a) Corey, E. J.; Guzman-Perez, A. Angew. Chem., Int. Ed. 1998, 37, 388. |
| [49] | (b) Quasdorf, K. W.; Overman, L. E. Nature 2014, 516, 181. |
| [49] | (c) Liu, Y.; Han, S. J.; Liu, W. B.; Stoltz, B. M. Acc. Chem. Res. 2015, 48, 740. |
| [49] | (d) Li, C.; Ragab, S. S.; Liu, G.; Tang, W. Nat. Prod. Rep. 2020, 37, 276. |
| [50] | (a) Arakawa, Y.; Fritz, S. P.; Wennemers, H. J. Org. Chem. 2014, 79, 3937. |
| [50] | (b) Musa, M. A.; Cooperwood, J. S.; Khan, M. O. F. Curr. Med. Chem. 2008, 15, 2664. |
| [50] | (c) Asai, F.; Iinuma, M.; Tanaka, T.; Takenaka, M.; Mizuno, M. Phytochemistry 1992, 31, 2487. |
| [51] | Sridharan, V.; Suryavanshi, P. A.; Menéndez, J. C. Chem. Rev. 2011, 111, 7157. |
| [52] | Engl, O. D.; Fritz, S. P.; K?slin, A.; Wennemers, H. Org. Lett. 2014, 16, 5454. |
| [53] | Cosimi, E.; Saadi, J.; Wennemers, H. Org. Lett. 2016, 18, 6014. |
| [54] | (a) He, M.; Uc, G. J.; Bode, J. W. J. Am. Chem. Soc. 2006, 128, 15088. |
| [54] | (b) Kaeobamrung, J.; Mahatthananchai, J.; Zheng, P.; Bode, J. W. J. Am. Chem. Soc. 2010, 132, 8810. |
| [54] | (c) Belmessieri, D.; Morrill, L. C.; Simal, C.; Slawin, A. M.; Smith, A. D. J. Am. Chem. Soc. 2011, 133, 2714. |
| [54] | (d) Mahatthananchai, J.; Kaeobamrung, J.; Bode, J. W. ACS Catal. 2012, 2, 494. |
| [54] | (e) Morrill, L. C.; Douglas, J.; Lebl, T.; Slawin, A. M.; Fox, D. J.; Smith, A. D. Chem. Sci. 2013, 4, 4146. |
| [54] | (f) Yao, W.; Dou, X.; Lu, Y. J. Am. Chem. Soc. 2015, 137, 54. |
| [54] | (g) Young, C. M.; Stark, D. G.; West, T. H.; Taylor, J. E.; Smith, A. D. Angew. Chem., Int. Ed. 2016, 55, 14394. |
| [54] | (h) Zhang, M. L.; Wu, Z. J.; Zhao, J. Q.; Luo, Y.; Xu, X. Y.; Zhang, X. M.; Yuan, W. C. Org. Lett. 2016, 18, 5110. |
| [54] | (i) Xu, D.; Zhang, Y.; Ma, D. Tetrahedron Lett. 2010, 51, 3827. |
| [54] | (j) Sinha, D.; Perera, S.; Zhao, J. C. G. Chem. Eur. J. 2013, 19, 6976. |
| [55] | Jin, H.; Lee, J.; Shi, H.; Lee, J. Y.; Yoo, E. J.; Song, C. E.; Ryu, D. H. Org. Lett. 2018, 20, 1584. |
| [56] | Bahlinger, A.; Fritz, S. P.; Wennemers, H. Angew. Chem., Int. Ed. 2014, 53, 8779. |
| [57] | Cosimi, E.; Engl, O. D.; Wennemers, H. Angew. Chem., Int. Ed. 2016, 55, 13127. |
| [58] | (a) Mitsunuma, H.; Shibasaki, M.; Kanai, M. Angew. Chem., Int. Ed. 2012, 51, 5217. |
| [58] | (b) Shimizu, Y.; Kanai, M.; Shibasaki, M. Angew. Chem., Int. Ed. 2010, 49, 1103. |
| [58] | (c) Overman, L. E.; Paone, D. V.; Stearns, B. A. J. Am. Chem. Soc. 1999, 121, 7702. |
| [58] | (d) Lemieux, R. M.; Meyers, A. I. J. Am. Chem. Soc. 1998, 120, 5453. |
| [59] | Liu, T.; Liu, W.; Shao, Z. J. Org. Chem. 2015, 80, 4950. |
| [60] | Engl, D. O.; Fritz, S. P.; Wennemers, H. Angew. Chem., Int. Ed. 2015, 54, 8193. |
| [61] | (a) Trost, B. M.; Chung, C. K.; Pinkerton, A. B. Angew. Chem., Int. Ed. 2004, 43, 4327. |
| [61] | (b) Fleming, J. J.; Du Bois, J. J. Am. Chem. Soc. 2006, 128, 3926. |
| [62] | Wang, Y.; Mo, M.; Zhu, K.; Zheng, C.; Zhang, H.; Wang, W.; Shao, Z. Nat. Commun. 2015, 6, 8544. |
| [63] | Ye, W.; Jiang, Z.; Zhao, Y.; Goh, S. L. M.; Leow, D.; Soh, Y.-T.; Tan, C.-H. Adv. Synth. Catal. 2007, 349, 2454. |
| [64] | Jiang, Z.; Yang, Y.; Pan, Y.; Zhao, Y.; Liu, H.; Tan, C.-H. Chem. Eur. J. 2009, 15, 4925. |
| [65] | Xu, J.; Fu, X.; Low, R.; Goh, Y.-P.; Jiang, Z.; Tan, C.-H. Chem. Commun. 2008, 5526. |
| [66] | (a) Okino, T.; Hoashi, Y.; Takemoto, Y. J. Am. Chem. Soc. 2003, 125, 12672. |
| [66] | (b) Okino, T.; Hoashi, Y.; Furukawa, T.; Xu, X.; Takemoto, Y. J. Am. Chem. Soc. 2005, 127, 119. |
| [67] | Jin, H.; Kim, S. T.; Hwang, G.-S.; Ryu, D. H. J. Org. Chem. 2016, 81, 3263. |
| [68] | (a) Sim, J. H.; Song, C. E. Angew. Chem., Int. Ed. 2017, 56, 1835. |
| [68] | (b) Klijn, J. E.; Engberts, J. B. F. N. Nature 2005, 435, 746. |
| [68] | (c) Jung, Y.; Marcus, R. A. J. Am. Chem. Soc. 2007, 129, 5492. |
| [68] | (d) Guo, W.; Liu, X.; Liu, Y.; Li, C. ACS Catal. 2018, 8, 328. |
| [68] | (e) Kitanosono, T.; Kobayashi, S. Chem. Eur. J. 2020, 26, 9408. |
| [68] | (f) Cortes-Clerget, M.; Yu, J.; Kincaid, J. R. I.; Walde, P.; Gallou, F.; Lipshutz, B. H. Chem. Sci. 2021, 12, 4237. |
| [69] | Sim, J. H.; Park, J. H.; Maity, P.; Song, C. E. Org. Lett. 2019, 21, 6715. |
| [70] | (a) Akiyama, T.; Itoh, J.; Yokota, K.; Fuchibe, K. Angew. Chem., Int. Ed. 2004, 43, 1566. |
| [70] | (b) Uraguchi, D.; Terada, M. J. Am. Chem. Soc. 2004, 126, 5356. |
| [70] | (c) Wu, X.; Li, M.; Gong, L. Acta Chim. Sinica 2013, 71, 1091. (in Chinese) |
| [70] | ( 吴祥, 李明丽, 龚流柱, 化学学报 2013, 71, 1091.) |
| [71] | Hatano, M.; Moriyama, K.; Maki, T.; Ishihara, K. Angew. Chem., Int. Ed. 2010, 49, 3823. |
| [72] | Song, J.; Shih, H.-W.; Deng, L. Org. Lett. 2007, 9, 603. |
| [73] | Bae, H. Y.; Kim, M. J.; Sim, J. H.; Song, C. E. Angew. Chem., Int. Ed. 2016, 55, 10825. |
| [74] | Gulevich, A. V.; Zhdanko, A. G.; Orru, R. V. A.; Nenajgenko, V. G. Chem. Rev. 2010, 110, 5235. |
| [75] | Odriozola, A.; Oiarbide, M.; Palomo, C. Chem. Eur. J. 2017, 23, 12758. |
| [76] | Oh, J.-S.; Lee, J.-W.; Ryu, T. H.; Lee, J. H.; Song, C. E. Org. Biomol. Chem. 2012, 10, 1052. |
| [77] | Yoshida, Y.; Kasuya, R.; Mino, T.; Sakamoto, M. Org. Biomol. Chem. 2021, 19, 6402. |
| [78] | (a) Nishimura, K.; Ono, M.; Nagaoka, Y.; Tomioka, K. J. Am. Chem. Soc. 1997, 119, 12974. |
| [78] | (b) Nishimura, K.; Tomioka, K. J. Org. Chem. 2002, 67, 431. |
| [78] | (c) Dong, X.-Q.; Fang, X.; Wang, C.-J. Org. Lett. 2011, 13, 4426. |
| [78] | (d) Fang, X.; Li, J.; Wang, C.-J. Org. Lett. 2013, 15, 3448. |
| [79] | Rigby, C. L.; Dixon, D. J. Chem. Commun. 2008, 39, 3798. |
| [80] | Destro, D.; Bottinelli, C.; Ferrari, L.; Albanese, D. C. M.; Bencivenni, G.; Gillick-Healy, M. W.; Adamo, M. F. A. J. Org. Chem. 2020, 85, 5183. |
| [81] | Maddocks, C. J.; Ermanis, K.; Clarke, P. A. Org. Lett. 2020, 22, 8116. |
| [82] | Fukata, Y.; Okamura, T.; Asano, K.; Matsubara, S. Org. Lett. 2014, 16, 2184. |
| [83] | Ahlemeyer, N. A.; Birman, V. B. Org. Lett. 2016, 18, 3454. |
| [84] | Ahlemeyer, N. A.; Streff, E. V.; Muthupandi, P.; Birman, V. B. Org. Lett. 2017, 19, 6486. |
| [85] | Lu, H.; Zhang, J.-L.; Liu, J.-Y.; Li, H.-Y.; Xu, P.-F. ACS Catal. 2017, 7, 7797. |
| [86] | (a) Das, J. P.; Marek, I. Chem. Commun. 2011, 47, 4593. |
| [86] | (b) Minko, Y.; Pasco, M.; Lercher, L.; Botoshansky, M.; Marek, I. Nature 2012, 490, 522. |
| [87] | Giacalone, F.; Gruttaduria, M.; Agrigento, P.; Noto, R. Chem. Soc. Rev. 2012, 41, 2406. |
/
| 〈 |
|
〉 |