

葫芦状有机金属配位笼的合成及其对两种药物分子的选择性结合
A Gourd-shaped Organometallic Coordination Cage: Synthesis and Selective Binding of Two Drug Molecules
Received date: 2022-12-27
Online published: 2023-03-01
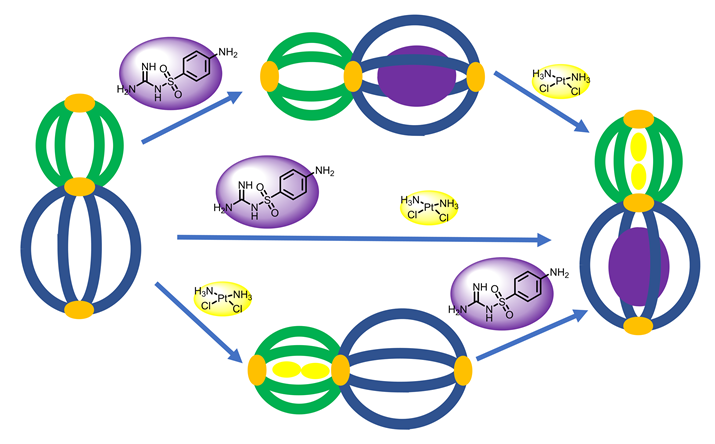
自1998年第一个设计并合成的Pd2L4型配位笼分子被报道以来, Pd2L4型笼被不断深入研究, 目前已广泛应用到催化、药物传递、分子识别等领域. 本工作介绍了一个葫芦状Pd2L4型有机金属配位笼(C1)的合成, 且经核磁共振氢谱(1H NMR)和高分辨质谱(HR-MS)表征, 并用1H NMR研究了C1与两种药物分子磺胺胍(G1)和顺铂(G2)的主客体化学行为. 1H NMR结果显示C1的两个不同空腔内能够选择性分别结合G1和G2. 无论G1和G2是同时加入还是分步加入, 都能得到相同的主客体复合物. 这一实验结果为后续的靶向给药相关研究奠定了基础, 为联合用药提供了新思路.
黄秀清 , 张琦 . 葫芦状有机金属配位笼的合成及其对两种药物分子的选择性结合[J]. 化学学报, 2023 , 81(3) : 217 -221 . DOI: 10.6023/A22120511
The Pd2L4 type coordination cage has been extensively studied and widely applied in catalysis, drug delivery, molecular recognition and other research fields since the seminal report in 1998. In this work, a gourd-shaped Pd2L4 type coordination cage (C1) was synthesized and characterized by proton nuclear magnetic resonance spectroscopy (1H NMR) and high resolution mass spectrum (HR-MS). The host-guest chemistry of C1 was studied by 1H NMR with two drug molecules, sulfaguanidine (G1) and cisplatin (G2). 1H NMR results show that G1 and G2 can be selectively combined in two different cavities of C1. The same host-guest complex could be obtained, whether G1 and G2 were added at the same time or in a stepwise fashion. This work laid a foundation for drug delivery and provided a new inspiration for concomitant drugs.

| [1] | (a) Li M.; Jiang S.; Zhang Z.; Hao X.; Jiang X.; Yu H.; Wang P.; Xu B.; Wang M.; Tian W. CCS Chemistry 2020, 2, 337. |
| [1] | (b) Zhang Z.; Ma L.; Fang F.; Hou Y.; Lu C.; Mu C.; Zhang Y.; Liu H.; Gao K.; Wang M.; Zhang Z.; Li X.; Zhang M. JACS Au 2022, 2, 1479. |
| [1] | (c) Wang H.; Li Y.; Li N.; Filosa A.; Li X. Nat. Rev. Mater. 2020, 6, 145. |
| [1] | (d) Wang H.; Guo C.; Li X. CCS Chemistry 2022, 4, 785. |
| [1] | (e) Shi J.; Li Y.; Jiang X.; Yu H.; Li J.; Zhang H.; Trainer D. J.; Hla S. W.; Wang H.; Wang M.; Li X. J. Am. Chem. Soc. 2021, 143, 1224. |
| [1] | (f) Pan M.; Wu K.; Zhang J.; Su C. Coord. Chem. Rev. 2019, 378, 333. |
| [1] | (g) Mai H. D.; Tran N. M.; Yoo H. Coord. Chem. Rev. 2019, 387, 180. |
| [1] | (h) Li Y.; Wang H.; Li X. Chem. Sci. 2020, 11, 12249. |
| [1] | (i) Lee S.; Jeong H.; Nam D.; Lah M. S.; Choe W. Chem. Soc. Rev. 2021, 50, 528. |
| [2] | McMorran D. A.; Steel P. J. Angew. Chem. Int. Ed. 1998, 37, 3295. |
| [3] | (a) Wang J.; Young T. A.; Duarte F.; Lusby P. J. J. Am. Chem. Soc. 2020, 142, 17743. |
| [3] | (b) Spicer R. L.; Stergiou A. D.; Young T. A.; Duarte F.; Symes M. D.; Lusby P. J. J. Am. Chem. Soc. 2020, 142, 2134. |
| [4] | (a) Kaiser F.; Schmidt A.; Heydenreuter W.; Altmann P. J.; Casini A.; Sieber S. A.; Kühn F. E. Eur. J. Inorg. Chem. 2016, 2016, 5189. |
| [4] | (b) Lewis J. E. M.; Gavey E. L.; Cameron S. A.; Crowley J. D. Chem. Sci. 2012, 3, 778. |
| [4] | (c) Schmidt A.; Molano V.; Hollering M.; Pothig A.; Casini A.; Kuhn F. E. Chem 2016, 22, 2253. |
| [5] | (a) Sumida R.; Tanaka Y.; Niki K.; Sei Y.; Toyota S.; Yoshizawa M. Chem. Sci. 2021, 12, 9946. |
| [5] | (b) Yamashina M.; Tsutsui T.; Sei Y.; Akita M.; Yoshizawa M. Sci. Adv. 2019, 5, eaav3179. |
| [5] | (c) Niki K.; Tsutsui T.; Yamashina M.; Akita M.; Yoshizawa M. Angew. Chem. Int. Ed. 2020, 59, 10489. |
| [6] | Preston D.; White K. F.; Lewis J. E. M.; Vasdev R. A. S.; Abrahams B. F.; Crowley J. D. Chem 2017, 23, 10559. |
| [7] | (a) Lewis J. E. M.; Tarzia A.; White A. J. P.; Jelfs K. E. Chem. Sci. 2019, 11, 677. |
| [7] | (b) Yu H.; Li J.; Shan C.; Lu T.; Jiang X.; Shi J.; Wojtas L.; Zhang H.; Wang M. Angew. Chem. Int. Ed. 2021, 60, 26523. |
| [8] | Lisboa L. S.; Findlay J. A.; Wright L. J.; Hartinger C. G.; Crowley J. D. Angew. Chem. Int. Ed. 2020, 59, 11101. |
| [9] | (a) Preston D.; Barnsley J. E.; Gordon K. C.; Crowley J. D. J. Am. Chem. Soc. 2016, 138, 10578. |
| [9] | (b) Johnson A. M.; Moshe O.; Gamboa A. S.; Langloss B. W.; Limtiaco J. F.; Larive C. K.; Hooley R. J. Inorg. Chem. 2011, 50, 9430. |
| [9] | (c) Liu Y.; Liao S.; Dai W.; Bai Q.; Lu S.; Wang H.; Li X.; Zhang Z.; Wang P.; Lu W.; Zhang Q. Angew. Chem. Int. Ed. 2022, 61, e202217215. |
| [10] | Zhu R.; Regeni I.; Holstein J. J.; Dittrich B.; Simon M.; Prevost S.; Gradzielski M.; Clever G. H. Angew. Chem. Int. Ed. 2018, 57, 13652. |
| [11] | Yazaki K.; Akita M.; Prusty S.; Chand D. K.; Kikuchi T.; Sato H.; Yoshizawa M. Nat. Commun. 2017, 8, 15914. |
| [12] | Yamaguchi T.; Tashiro S.; Tominaga M.; Kawano M.; Ozeki T.; Fujita M. J. Am. Chem. Soc. 2004, 126, 10818. |
| [13] | Samantray S.; Krishnaswamy S.; Chand D. K. Nat. Commun. 2020, 11, 880. |
| [14] | Preston D.; Lewis J. E.; Crowley J. D. J. Am. Chem. Soc. 2017, 139, 2379. |
| [15] | Lisboa L. S.; Preston D.; McAdam C. J.; Wright L. J.; Hartinger C. G.; Crowley J. D. Angew. Chem. Int. Ed. 2022, 61, e202201700. |
| [16] | Lewis J. M. Angew. Chem. Int. Ed. 2022, 61, e202212392. |
| [17] | O'Connor, H. M.; Tipping, W. J.; Vallejo, J.; Nichol, G. S.; Faulds, K.; Graham, D.; Brechin, E. K.; Lusby, P. J. Inorg. Chem. 2022,10.1021/acs.inorgchem.2c00873. |
| [18] | (a) Liu Q. L.; Fang P. J.; Zhao Z. L.; Zhang H. Z.; Zhou C. H. Chin. J. Org. Chem. 2017, 37, 3146. (in Chinese) |
| [18] | (刘庆龙, 房鹏金, 赵志龙, 张慧珍, 周成合, 有机化学, 2017, 37, 3146). |
| [18] | (b) Zhang H. Z.; He S. C.; Peng Y. J.; Zhang H. J.; Gopala L.; Tangadanchu V. K. R.; Gan L. L.; Zhou C. H. Eur. J. Med. Chem. 2017, 136, 165. |
| [19] | Kelland L. Nat. Rev. Cancer. 2007, 7, 573. |
/
| 〈 |
|
〉 |