

一种热响应介电开关型零维有机-无机杂化材料: (C3H6NH2)2CoCl4
收稿日期: 2023-03-04
网络出版日期: 2023-04-27
基金资助
受国家自然科学基金(22201134); 江苏省高校自然科学基金(22KJB150028)
A Thermally Responsive Dielectric Switchable Zero-Dimensional Organic-Inorganic Hybrid Material: (C3H6NH2)2CoCl4
Received date: 2023-03-04
Online published: 2023-04-27
Supported by
National Natural Science Foundation of China(22201134); Natural Science Foundation for Colleges and Universities of Jiangsu Province(22KJB150028)
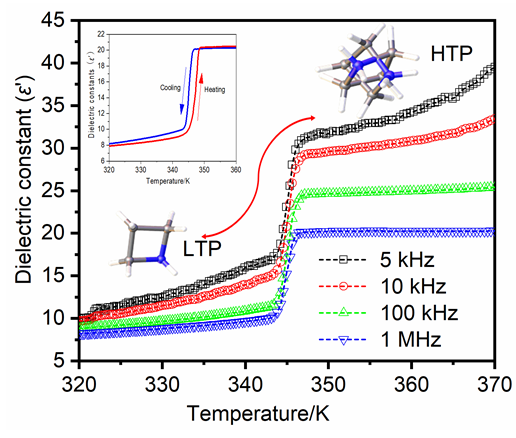
刺激响应型智能材料, 因其制备简单、结构多变、成本低廉, 在信息存储和传感器等方面有着广阔的应用前景. 本工作通过溶剂挥发法成功制备了一例新型零维有机-无机杂化材料: (C3H6NH2)2CoCl4. 差示扫描量热(DSC)和变温介电测试结果表明, (C3H6NH2)2CoCl4在347.7 K处发生可逆的结构相变, 并伴随着明显的“台阶状”介电异常, 表现出优良的介电开关特性. 并通过变温单晶X-射线衍射(SCXRD)对相变机理进行研究, 发现其经历了从P21/n空间群转变为Pnma空间群的结构变化, 这主要源于(C3H6NH2)+阳离子剧烈的有序-无序运动和(CoCl4)2-阴离子微小的畸变作用. 此外, 本工作通过Crystal Explorer计算了阳离子周围的弱相互作用, 并发现了阳离子之间的H•••H相互作用和阴、阳离子之间的H•••Cl相互作用是形成该杂化材料的主要原因. 紫外-可见吸收光谱显示, 该化合物的吸收截止边为743 nm, 其禁带宽度Eg为4 eV.
陈剑 , 蔡卓尔 , 焦淑琳 , 张祥 , 胡进忠 , 刘敏 , 孙伯旺 , 花秀妮 . 一种热响应介电开关型零维有机-无机杂化材料: (C3H6NH2)2CoCl4[J]. 化学学报, 2023 , 81(5) : 480 -485 . DOI: 10.6023/A23030064
Stimuli-responsive smart materials have garnered significant interest due to their potential applications in information storage and sensors. These materials possess a simple preparation process, versatile structure, and are cost-effective, particularly the organic-inorganic hybrid-type thermally responsive dielectric switching materials. To this end, we selected azetidine hydrochloride and cobalt chloride hexahydrate and utilized a straightforward solvent evaporation method to obtain blue transparent crystals, (C3H6NH2)2CoCl4, a zero-dimensional organic-inorganic hybrid material exhibiting temperature responsiveness. It is notable that this compound undergoes a reversible structural phase transition at 347.7 K. And the enthalpy change of the material was consistent, as revealed by the differential scanning calorimetry (DSC) curves tested at different ramp-up and ramp-down rates. The heat absorption and exothermic peaks also displayed consistency at different rates, with the peaks demonstrating a tendency to contract. The crystal structure of the material underwent a phase transition from P21/n to Pnma space group, as evidenced by single crystal X-ray diffraction (SCXRD) data at variable temperatures. This transition was attributed to the deformation and displacement of the (CoCl4)2- anions and the ordered-disordered movements of the (C3H6NH2)+ cation. The dielectric test results of (C3H6NH2)2CoCl4 exhibited a step-like dielectric anomaly, with consistent dielectric changes over several dielectric cycles, demonstrating excellent dielectric switching properties. Weak interactions around the cations were calculated by Crystal Explorer, indicating that the main cause of the hybrid material formation was due to H•••H interactions between the cations and H•••Cl interactions between the anions and cations. Additionally, based on the ultraviolet-visible absorption spectroscopy, (C3H6NH2)2CoCl4 displayed an absorption edge at 743 nm, with an estimated optical bandgap Eg of 4 eV. Moreover, the material demonstrated exceptional stability, cycle life, and durability, indicating its potential for a wide range of applications in energy storage, sensors, catalysts, and other fields. In conclusion, this work provides novel insights into the structural design and property modulation of organic-inorganic hybrid materials.

| [1] | Kang, S.; Jillella, R.; Park, S.; Park, S.; Kim, J. H.; Oh, D.; Kim, J.; Park, J. Nanomaterials 2022, 12, 3806. |
| [2] | Jia, Q. Q.; Ni, H. F.; Lun, M. M.; Xie, L. Y.; Lu, H. F.; Fu, D. W.; Guo, Q. J. Mater. Chem. C 2022, 10, 16330. |
| [3] | Bai, T. X.; Wang, X. C.; Wang, Z. Y.; Ji, S. J.; Meng, X.; Wang, Q. J.; Zhang, R. L.; Han, P. G.; Han, K. L.; Chen, J. S.; Liu, F.; Yang, B. Angew. Chem. Int. Ed. 2023, 62, e202213240. |
| [4] | Liu, S.; He, L.; Wang, Y.; Shi, P.; Ye, Q. Chinese Chem. Lett. 2022, 33, 1032. |
| [5] | Fukuta, Y.; Miyata, T.; Hamanaka, Y. J. Mater. Chem. C 2023, 11, 910. |
| [6] | Tian, Y.; Peng, H.; Wei, Q. L.; Chen, Y. X.; Xia, J. J.; Lin, W. C.; Peng, C. Y.; He, X. F.; Zou, B. S. Chem. Eng. J. 2023, 458, 141436. |
| [7] | Liu, H.-Y.; Zhang, H.-Y.; Chen, X.-G.; Xiong, R.-G. J. Am. Chem. Soc. 2020, 142, 15205. |
| [8] | Wang, C. C.; Yan, R. Y.; Cai, M. J.; Liu, Y. P.; Li, S. J. Appl. Surf. Sci. 2023, 610, 155346. |
| [9] | Guo, Z.-Y.; Zhou, H.-P. Acta Chim. Sinica 2021, 79, 223. (in Chinese) |
| [9] | (郭镇域, 周欢萍, 化学学报, 2021, 79, 223.) |
| [10] | Xu, H.-J.; Han, S.-G.; Sun, Z.-H.; Luo, J.-H. Acta Chim. Sinica 2021, 79, 23. (in Chinese) |
| [10] | 徐豪杰, 韩世国, 孙志华, 罗军华, 化学学报, 2021, 79, 23.) |
| [11] | Xu, W.-J.; He, C.-T.; Ji, C.-M.; Chen, S.-L.; Huang, R.-K.; Lin, R.-B.; Xue, W.; Luo, J.-H.; Zhang, W.-X.; Chen, X.-M. Adv. Mater. 2016, 28, 5886. |
| [12] | Han, S.; Zhang, J.; Sun, Z.; Ji, C.; Zhang, W.; Wang, Y.; Tao, K.; Teng, B.; Luo, J. Inorg. Chem. 2017, 56, 13078. |
| [13] | Wu, Y. L.; Lu, S. H.; Zhou, Q. H.; Ju, M. G.; Zeng, X. C.; Wang, J. L. Adv. Funct. Mater. 2022, 32, 4579. |
| [14] | Wu, L. K.; Feng, Y.; Wang, Z. J.; Li, L. H.; Hu, Z. B.; Ye, H. Y.; Li, J. R. Inorg. Chem. Commun. 2022, 142, 109641. |
| [15] | Wu, F. F.; Wei, Q. Y.; Li, X. Q.; Liu, Y.; Huang, W. Q.; Chen, Q.; Li, B. X.; Luo, J. H.; Liu, X. T. Cryst. Growth Des. 2022, 22, 3875. |
| [16] | Rok, M.; Zarychta, B.; Janicki, R.; Witwicki, M.; Bienko, A.; Bator, G. Inorg. Chem. 2022, 61, 5626. |
| [17] | RaeisianAsl, M.; Panahi, S. F. K. S.; Jamaati, M.; Tafreshi, S. S. Int. J. Energ. Res. 2022, 46, 13117. |
| [18] | Liu, Y. H.; Wang, W. Q.; Zhang, B. L.; Wang, Y. J.; Ren, M. P.; Jing, Z. H.; Yue, C. Y. CrystEngComm 2023, 25, 444. |
| [19] | Hua, X.-N.; Huang, C.-R.; Gao, J.-X.; Lu, Y.; Chen, X.-G.; Liao, W.-Q. Dalton T. 2018, 47, 6218. |
| [20] | Liu, J. Y.; Ye, S. Y.; Wan, M.; Wang, Y. N.; Tong, L.; Chen, L. Z. New J. Chem. 2022, 46, 1054.. |
| [21] | Rao, W.; Li, M.; You, X.; Wei, Z.; Zhang, M.; Wang, L.; Cai, H. Inorg. Chem. 2021, 60, 14706. |
| [22] | Hua, X.-N.; Gao, J.-X.; Chen, X.-G.; Li, P.-F.; Mei, G.-Q.; Liao, W.-Q. Dalton T. 2019, 48, 6621. |
| [23] | Jayatilaka, D.; Wolff, S. K.; Grimwood, D. J.; McKinnon, J. J.; Spackman, M. A. Acta Cryst. 2006, 62, 1107. |
| [24] | Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Cryst. 2009, 42, 339. |
| [25] | Sheldrick, G. M. Acta Cryst. 2015, A71, 3. |
/
| 〈 |
|
〉 |