

柔性芴基嵌段型延迟荧光二聚体的设计、合成及电致发光性能
收稿日期: 2023-02-26
网络出版日期: 2023-05-23
基金资助
国家自然科学基金(21805106); 连云港科技成果转化项目(CA202103); 连云港521资助项目(LYG06521202161); 江苏省六大人才高峰项目(JNHB 114)
Design, Synthesis and Electroluminescence Performance of Flexible Fluorenyl Block Delayed Fluorescence Dimers
Received date: 2023-02-26
Online published: 2023-05-23
Supported by
The National Natural Science Foundation of China(21805106); Lianyungang Project for Transformation of Scientific and Technological Achievements(CA202103); Lianyungang 521 Funding Project(LYG06521202161); Six Talent Peaks Project in Jiangsu Province(JNHB 114)
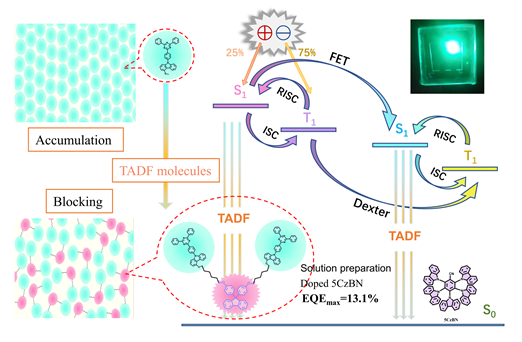
热激活延迟荧光(TADF)材料虽然可以通过反向系间窜越(RISC)利用三线态激子, 但紧密堆积状态下的三重态激子湮灭会严重降低器件效率. 本工作采用二苯基芴作为嵌段骨架, 三嗪-咔唑(TRZ-Cz)作为TADF发光单元合成了嵌段型二聚体材料2TC-Fu. 通过柔性烷基链将二苯基芴桥连在TADF的中间, 不仅有效避免了发光核的聚集猝灭, 还提高了其在常用溶剂中的溶解性, 使其可以通过溶液法制备有机发光二极管(OLEDs). 光物理和热力学测试表明, 2TC-Fu的三线态为2.89 eV, 且具有较小的ΔEST (0.27 eV)和较高的玻璃化转变温度(142 ℃). 以2TC-Fu作为非掺杂发光层, 溶液法器件的最大电流效率(CE)和外量子效率(EQEmax)分别为8.3 cd/A和4.4%, 几乎是未嵌段发光单元的5倍, 充分证明嵌段单元可以有效抑制激子猝灭. 将其作为主体材料, 掺杂器件实现了13.1%的EQEmax, 这在溶液法OLEDs中属于较高水平. 上述研究结果表明, 柔性芴基嵌段策略可以有效提高分子的电致发光效率, 为可溶液加工型TADF分子的开发提供了新的途径.
葛凤洁 , 张开志 , 曹清鹏 , 徐慧 , 周涛 , 张文浩 , 班鑫鑫 , 张晓波 , 李娜 , 朱鹏 . 柔性芴基嵌段型延迟荧光二聚体的设计、合成及电致发光性能[J]. 化学学报, 2023 , 81(9) : 1157 -1166 . DOI: 10.6023/A23020051
Organic light-emitting diodes (OLEDs) have a series of advantages such as high resolution, wide viewing angle, lightweight, and flexible solution processing that can realize a large area of self-illumination display. As the third generation of luminescence materials, thermally-activated delayed fluorescence (TADF) material can harvest 100% of triplet excitons through reverse intersystem crossing (RISC). However, the triplet excitons exhibit severe aggregation-caused quenching (ACQ) behavior in the tightly stacked state, which undoubtedly reduces the device’s performance. In this work, we synthesized the block dimer TADF material 2TC-Fu, among the molecular structure, diphenylfluorene serves as the block framework and triazinocarbazole (TRZ-CZ) serves as the TADF emissive unit. First, carbazole is connected to the end of 1,6-dibromohexane, and then the symmetric structure is synthesized from nucleophilic substituted bisphenol fluorene, after, monofluorotriazine is attached to both ends of the symmetric structure by a nucleophilic substitution reaction to generate the target product 2TC-Fu finally. Because the addition of diphenyl fluorene obviously shows a spatial torsion angle, the irregular distribution of the emitting unit is realized by bridging the flexible alkyl chain in the middle of TADF, thus effectively avoiding the ACQ of the TADF unit and improving its solubility in the solvent. Thermodynamic properties show that 2TC-Fu has a high glass transition temperature (142 ℃) and a thermal decomposition temperature of 442 ℃, proving that it can be sufficient to withstand the heat treatment procedures during device preparation. In addition, the device 2TC-Fu based exhibits a high CEmax and EQEmax of 8.3 cd/A and 4.4% respectively, which exceeds 3 times the efficiency of non-block TADF unit-based, demonstrating the feasibility of the flexible fluorenyl block strategy. Moreover, the devices 2TC-Fu doped with TADF material 5CzBN achieved 13.1% of EQEmax and 3489 cd/m2 of brightness. In summary, the design of a flexible fluorenyl block strategy to avoid large-scale aggregation of luminescent nuclei significantly improves the device efficiency, which provides a new way for the development of TADF molecules. Although the aggregation-induced fluorescence quench-ing was effectively inhibited by increasing the molecular spacing between luminescent units and increasing the solubility of molecules by using large fluorenyl groups and alkoxy chains, we will not stop researching the blocking strategy. In the future, we will continue to explore the factors that affect the performance of TADF devices in all aspects, such as the type of block groups and the steric hindrance of substituents.

| [1] | Wong, M. Y.; Zysman-Colman, E. Adv. Mater. 2017, 29, 1605444. |
| [2] | Krucaite, G.; Grigalevicius, S. Synth Met 2019, 247, 90. |
| [3] | Manikandan, M.; Nirmal, D.; Ajayan, J.; Mohankumar, P.; Prajoon, P.; Arivazhagan, L. Superlattices Microstruct 2019, 136, 106294. |
| [4] | Ràfols-Ribé, J.; Will, P.-A.; H?nisch, C.; Gonzalez-Silveira, M.; Lenk, S.; Rodríguez-Viejo, J.; Reineke, S. Sci. Adv. 2018, 4, eaar8332. |
| [5] | Zhang, L.; Li, M.; Gao, Q. Y.; Chen, C. F. Chin J. Org. Chem 2020, 40, 516. (in Chinese) |
| [5] | 张亮, 李猛, 高庆宇, 陈传峰, 有机化学, 2020, 40, 516). |
| [6] | Zhang, S. Q.; Li, M. Q.; Zhou, Z. J.; Qu, Z. X. Acta Chim Sinica 2023, 81, 124. (in Chinese) |
| [6] | 张少秦, 李美清, 周中军, 曲泽星, 化学学报, 2023, 81, 124). |
| [7] | Tan, H.-J.; Yang, G.-X.; Deng, Y.-L.; Cao, C.; Tan, J.-H.; Zhu, Z.-L.; Chen, W.-C.; Xiong, Y.; Jian, J.-X.; Lee, C.-S.; Tong, Q.-X. Adv. Mater. 2022, 34, 2200537. |
| [8] | Zhang, Q.; Li, B.; Huang, S.; Nomura, H.; Tanaka, H.; Adachi, C. Nat. Photonics 2014, 8, 326. |
| [9] | Ogorodnikov, I. N.; Kiseleva, M. S.; Yakovlev, V. Y. Opt. Mater. 2012, 34, 2030. |
| [10] | Vázquez, R. J.; Yun, J. H.; Muthike, A. K.; Howell, M.; Kim, H.; Madu, I. K.; Kim, T.; Zimmerman, P.; Lee, J. Y.; Iii, T. G. J. Am. Chem. Soc. 2020, 142, 8074. |
| [11] | Samanta, P. K.; Kim, D.; Coropceanu, V.; Brédas, J.-L. J. Am. Chem. Soc. 2017, 139, 4042. |
| [12] | Zhang, Q.; Li, J.; Shizu, K.; Huang, S.; Hirata, S.; Miyazaki, H.; Adachi, C. J. Am. Chem. Soc. 2012, 134, 14706. |
| [13] | Nakanotani, H.; Furukawa, T.; Morimoto, K.; Adachi, C. Sci. Adv. 2016, 2, e1501470. |
| [14] | Cravcenco, A.; Hertzog, M.; Ye, C.; Iqbal, M. N.; Mueller, U.; Eriksson, L.; B?rjesson, K. Sci. Adv. 2019, 5, eaaw5978. |
| [15] | Wang, J.; Li, N.; Chen, Q.; Xiang, Y.; Zeng, X.; Gong, S.; Zou, Y.; Liu, Y. Chem. Eng. J. 2022, 450, 137805. |
| [16] | Ikeda, N.; Oda, S.; Matsumoto, R.; Yoshioka, M.; Fukushima, D.; Yoshiura, K.; Yasuda, N.; Hatakeyama, T. Adv. Mater. 2020, 32, 2004072. |
| [17] | Rajamalli, P.; Senthilkumar, N.; Huang, P. Y.; Ren-Wu, C. C.; Lin, H. W.; Cheng, C. H. J. Am. Chem. Soc. 2017, 139, 10948. |
| [18] | Aizawa, N.; Matsumoto, A.; Yasuda, T. Sci. Adv. 2021, 7, eabe5769. |
| [19] | Phan Huu, D. K. A.; Saseendran, S.; Dhali, R.; Franca, L. G.; Stavrou, K.; Monkman, A.; Painelli, A. J. Am. Chem. Soc. 2022, 144, 15211. |
| [20] | Bezvikonnyi, O.; Gudeika, D.; Volyniuk, D.; Bucinskas, A.; Grazulevicius, J. V. Mater. Sci. Eng. B 2021, 273, 115441. |
| [21] | Fu, Y.; Liu, H.; Yang, D.; Ma, D.; Zhao, Z.; Tang, B. Z. Sci. Adv. 2021, 7, eabj2504. |
| [22] | Kim, E.; Park, J.; Jun, M.; Shin, H.; Baek, J.; Kim, T.; Kim, S.; Lee, J.; Ahn, H.; Sun, J.; Ko, S.-B.; Hwang, S.-H.; Lee, J. Y.; Chu, C.; Kim, S. Sci. Adv. 2022, 8, eabq1641. |
| [23] | Kawasumi, K.; Wu, T.; Zhu, T.; Chae, H. S.; Van Voorhis, T.; Baldo, M. A.; Swager, T. M. J. Am. Chem. Soc. 2015, 137, 11908. |
| [24] | Li, H.; Wang, Y.; Yu, L.; Liu, C.; Zhou, C.; Sun, S.; Li, M.; Tao, Y.; Xie, G.; Xu, H.; Huang, W.; Chen, R. Chem. Eng. J. 2021, 425, 131487. |
| [25] | Manivannan, R.; Park, S. H.; Oh, H.; Son, Y.-A. Dyes Pigm. 2022, 205, 110480. |
| [26] | Lv, X.; Wang, Y.; Li, N.; Cao, X.; Xie, G.; Huang, H.; Zhong, C.; Wang, L.; Yang, C. Chem. Eng. J. 2020, 402, 126173. |
| [27] | Li, N.; Chai, D.; Chen, Z.; Zhou, C.; Ni, F.; Huang, Z.; Cao, X.; Xie, G.; Li, K.; Yang, C. Chem. Eng. J. 2020, 396, 125276. |
| [28] | Liang, Z. P.; Tang, R.; Qiu, Y. C.; Wang, Y.; Lu, H. B.; Wu, Z. G. Acta Chim. Sinica 2021, 79, 1401. (in Chinese) |
| [28] | 梁志鹏, 唐瑞, 邱雨晨, 王阳, 陆洪彬, 吴正光, 化学学报, 2021, 79, 1401). |
| [29] | Tao, Y.; Yuan, K.; Chen, T.; Xu, P.; Li, H.; Chen, R.; Zheng, C.; Zhang, L.; Huang, W. Adv. Mater. 2014, 26, 7931. |
| [30] | Lee, S. Y.; Adachi, C.; Yasuda, T. Adv. Mater. 2016, 28, 4626. |
| [31] | Ha, T. H.; Bin, J.-K.; Lee, C. W. Org. Electron. 2022, 102, 106450. |
| [32] | Armakovi?, S. J.; Mary, Y. S.; Mary, Y. S.; Pelemi?, S.; Armakovi?, S. Comput. Theor. Chem. 2021, 1197, 113160. |
| [33] | Skuodis, E.; Bezvikonnyi, O.; Tomkeviciene, A.; Volyniuk, D.; Mimaite, V.; Lazauskas, A.; Bucinskas, A.; Keruckiene, R.; Sini, G.; Grazulevicius, J. V. Org. Electron. 2018, 63, 29. |
| [34] | Moon, C.-K.; Suzuki, K.; Shizu, K.; Adachi, C.; Kaji, H.; Kim, J.-J. Adv. Mater. 2017, 29, 1606448. |
| [35] | Yang, H.-Y.; Zhang, M.; Zhao, J.-W.; Pu, C.-P.; Lin, H.; Tao, S.-L.; Zheng, C.-J.; Zhang, X.-H. Chin. J. Chem. 2022, 40, 911. |
| [36] | Wang, T. T.; Hua, X. C.; Yu, Y. J.; Yuan, Y.; Fung, M. Q.; Jiang, Z. Q. Chin J. Org. Chem 2019, 39, 1436. (in Chinese) |
| [36] | 王彤彤, 华晓晨, 郁友军, 袁熠, 冯敏强, 蒋佐权, 有机化学, 2019, 39, 1436). |
| [37] | Hong, G.; Gan, X.; Leonhardt, C.; Zhang, Z.; Seibert, J.; Busch, J. M.; Br?se, S. Adv. Mater. 2021, 33, 2005630. |
| [38] | Liu, Y.; Li, C.; Ren, Z.; Yan, S.; Bryce, M. R. Nat. Rev. Mater. 2018, 3, 18020. |
| [39] | Cao, X.; Chen, Z.; Gong, S.; Pan, K.; Zhou, C.; Huang, T.; Chai, D.; Zhan, Q.; Li, N.; Zou, Y.; Liu, H.; Yang, C. Chem. Eng. J. 2020, 399, 125648. |
| [40] | Al-Otaibi, J. S.; Mary, Y. S.; Mary, Y. S.; Soman, S.; Acharjee, N.; Narayana, B. Chem. Phys. Lett. 2022, 793, 139469. |
| [41] | Yersin, H.; Czerwieniec, R.; Monkowius, U.; Ramazanov, R.; Valiev, R.; Shafikov, M. Z.; Kwok, W.-M.; Ma, C. Coord. Chem. Rev. 2023, 478, 214975. |
| [42] | Sun, D.; Duda, E.; Fan, X.; Saxena, R.; Zhang, M.; Bagnich, S.; Zhang, X.; Kohler, A.; Zysman-Colman, E. Adv. Mater. 2022, 34, e2110344. |
| [43] | Wei, J.; Liu, D.; Sun, K.; Tian, W.; Jiang, W.; Sun, Y. Dyes Pigm. 2020, 182, 108624. |
| [44] | Liu, B.; Li, J.; Liu, D.; Mei, Y.; Lan, Y.; Song, K.; Li, Y.; Wang, J. Dyes Pigm. 2022, 203, 110329. |
| [45] | Shin, K.; Lee, E.; Lee, T.; Lee, Y. H.; Kim, D. H.; Kim, C.; Jung, J.; Jung, B. J.; Lee, M. H. Dyes Pigm. 2023, 209, 110937. |
| [46] | Chen, Y.; Li, N.; Huang, Z.; Xie, G.; Yang, C. Chem. Eng. J. 2022, 430, 133078. |
| [47] | Wang, T.-T.; Xie, G.; Li, H.-C.; Yang, S.-Y.; Li, H.; Xiao, Y.-L.; Zhong, C.; Sarvendra, K.; Khan, A.; Jiang, Z.-Q.; Liao, L.-S. CCS Chemistry 2021, 3, 1757. |
| [48] | Zhang, K.; Zhou, T.; Cao, Q.; Ge, F.; Xu, H.; Chu, J.; Wang, J.; Pei, M.; Ban, X.; Zhang, T. Org. Electron. 2023, 112, 106687. |
| [49] | Pathak, S. K.; Liu, H.; Zhou, C.; Xie, G.; Yang, C. J. Mater. Chem. C 2021, 9, 7363. |
| [50] | Zhao, G.; Liu, D.; Wang, P.; Huang, X.; Chen, H.; Zhang, Y.; Zhang, D.; Jiang, W.; Sun, Y.; Duan, L. Angew. Chem. Int. Ed. 2022, 61, e202212861. |
| [51] | Liang, J.-J.; Li, Y.; Yuan, Y.; Li, S.-H.; Zhu, X.-D.; Barlow, S.; Fung, M.-K.; Jiang, Z.-Q.; Marder, S. R.; Liao, L.-S. Mater. Chem. Front. 2018, 2, 917. |
| [52] | Niu, R.; Li, J.; Liu, D.; Dong, R.; Wei, W.; Tian, H.; Shi, C. Dyes Pigm. 2021, 194, 109581. |
| [53] | Li, Y.; Liang, J. J.; Li, H. C.; Cui, L. S.; Fung, M. K.; Barlow, S.; Marder, S. R.; Adachi, C.; Jiang, Z. Q.; Liao, L. S. J. Mater. Chem. C 2018, 6, 5536. |
/
| 〈 |
|
〉 |