

不对称α-二亚胺镍催化制备聚烯烃弹性体★
收稿日期: 2023-04-25
网络出版日期: 2023-06-02
基金资助
国家自然科学基金(52025031); 国家自然科学基金(U19B6001); 国家自然科学基金(U1904212)
Catalytic Synthesis of Polyolefin Elastomer Using Unsymmetrical α-Diimine Nickel Catalyst★
Received date: 2023-04-25
Online published: 2023-06-02
Supported by
National Natural Science Foundation of China(52025031); National Natural Science Foundation of China(U19B6001); National Natural Science Foundation of China(U1904212)
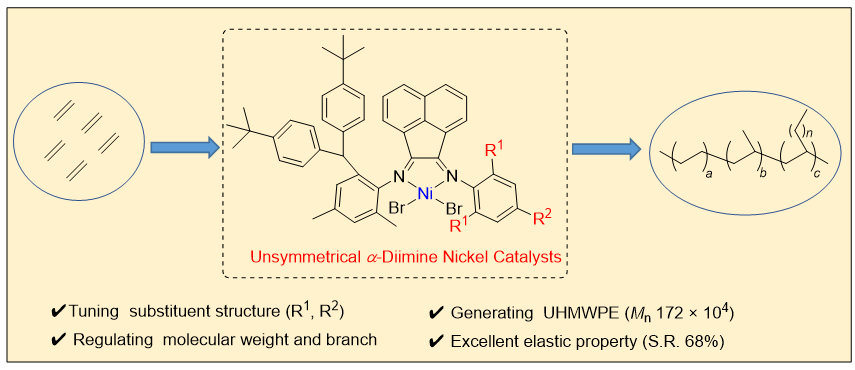
自齐格勒-纳塔催化剂被发现至今, 烯烃聚合领域在催化剂的设计方面已经取得了巨大的突破, 但是, 催化剂的发展依然是此领域新鲜的源泉. 通过催化剂的结构设计来影响烯烃聚合过程将一直是此领域前沿的科学问题. 由于金属镍低廉的价格以及较高的丰度使其在烯烃聚合中具有广泛的应用前景. 设计了三种具有不同位阻效应的不对称α-二亚胺镍催化剂, 并探究了其在乙烯聚合过程中的催化性能. 研究发现, 催化剂的种类以及聚合条件的变化对于乙烯聚合活性, 所制备聚乙烯的分子量、支化度、热力学参数以及力学性能都具有重要的影响. 其中大位阻的催化剂Ni3所催化制备的聚烯烃为超高分子量聚乙烯, 并同时保持了优良的力学性能以及弹性性能.
王子豪 , 陈敏 , 陈昶乐 . 不对称α-二亚胺镍催化制备聚烯烃弹性体★[J]. 化学学报, 2023 , 81(6) : 559 -564 . DOI: 10.6023/A23040162
Since the discovery of Ziegler-Natta catalyst, great breakthrough has been made in the design of catalyst in olefin polymerization field, but the development of catalyst is still the fresh source in this field. It will always be a frontier scientific problem in this field to influence the olefin polymerization process through the structural design of catalysts. Due to the low cost of nickel and its high abundance, nickel-based catalysts have shown great prospects in the application of olefin polymerization. In this work, three kinds of unsymmetrical α-diimine nickel catalysts with different steric effects were designed, and their catalytic performances in ethylene polymerization were explored. It is found that the variety of catalysts and the change of polymerization conditions have important effects on the catalytic activity of ethylene polymerization, the molecular weight, branching density, thermodynamic parameters and mechanical properties of the prepared polyethylene. Among them, the polyolefin prepared by Ni3 catalyst with large steric hindrance is ultra-high molecular weight polyethylene, and the above materials maintain excellent mechanical properties and elastic properties at the same time. Specifically, tert-butyl substituted ketoimine intermediate B was condensed with three different anilines to synthesize ligands L1~L3 with different steric effects. The catalysts Ni1~Ni3 were successfully synthesized by efficient coordination reaction between the obtained ligands and DMENiBr2, and the yields were over 90%. The single crystal structures of catalysts Ni1 and Ni3 were also prepared, and the steric hindrance around the nickel center was quantified utilizing the steric maps [Vbur(Ni1)=46.1%, Vbur(Ni3)=48.7%]. Under 0.8 MPa pressure of ethylene and AlEt2Cl as cocatalyst, the ethylene polymerization performance was explored using three nickel catalysts at different temperatures. Among them, Ni2 exhibited the best catalytic activity in ethylene polymerization at 50 ℃ (up to 1.7×107 g•mol-1•h-1), generating polyethylene with higher branching density. The polyethylene prepared by Ni3 with large steric hindrance is ultra-high molecular weight polyethylene with a molecular weight of 172×104 g•mol-1. Moreover, polyethylene materials prepared by Ni3 also showed excellent mechanical properties (breaking stress, 6~8 MPa; elongation at break, ≈600%) and elastic properties (strain recovery value up to 68%).

| [1] | Sturzel, M.; Mihan, S.; Mulhaupt, R. Chem. Rev. 2016, 116, 1398. |
| [2] | Cui, D. M. Acta Polym. Sinica 2020, 51, 12. (in Chinese) |
| [2] | (崔冬梅, 高分子学报, 2020, 51, 12.) |
| [3] | Jian, Z. B. Acta Polym. Sinica 2018, (11), 1359. (in Chinese) |
| [3] | (简忠保, 高分子学报, 2018, (11), 1359.) |
| [4] | Chen, M.; Chen, C. L. Acta Polym. Sinica 2018, (11), 1372. (in Chinese) |
| [4] | (陈敏, 陈昶乐, 高分子学报, 2018, (11), 1372.) |
| [5] | Tan, C.; Chen, C. L. Angew. Chem., Int. Ed. 2019, 58, 7192. |
| [6] | Li, Y.; Wang, X. Y.; Tang, Y. Acta Chim. Sinica 2021, 79, 1320. (in Chinese) |
| [6] | (李勇, 王晓艳, 唐勇, 化学学报, 2021, 79, 1320.) |
| [7] | Peng, W.; Qi, P. Y.; Dong, K. X.; He, A. H. Acta Chim. Sinica 2020, 78, 1418. (in Chinese) |
| [7] | (彭伟, 戚佩瑶, 董凯旋, 贺爱华, 化学学报, 2020, 78, 1418.) |
| [8] | Mu, H.; Pan, L.; Li, Y. Chem. Rev. 2015, 115, 12091. |
| [9] | Mu, H. L.; Zhou, G. L.; Hu, X. Q.; Jian, Z. B. Coord. Chem. Rev. 2021, 435, 213802. |
| [10] | Chen, C. Nat. Rev. Chem. 2018, 2, 6. |
| [11] | Fu, L. R.; Wang, Y. B.; Jiang, H.; Hao, X. Q.; Song, M. P. Chin. J. Org. Chem. 2022, 42, 3530. (in Chinese) |
| [11] | (付联荣, 王艳冰, 姜辉, 郝新奇, 宋毛平, 有机化学, 2022, 42, 3530.) |
| [12] | Wang, Y.; Yan, J. L. Acta Chim. Sinica 2023, 81, 275. (in Chinese) |
| [12] | (汪阳, 阎敬灵, 化学学报, 2023, 81, 275.) |
| [13] | Zhang, Y. X.; Zhang, Y. X.; Hu, X. Q.; Wang, C. Q.; Jian, Z. B. ACS Catal. 2022, 12, 14304. |
| [14] | Wang, H. B.; Yang, Y.; Nishiura, M.; Higaki, Y.; Takahara, A.; Hou, Z. M. J. Am. Chem. Soc. 2019, 141, 3249. |
| [15] | Wu, Y.; Nan, T. H.; Ji, X. L.; Liu, B.; Cui, D. M. Angew. Chem., Int. Ed. 2022, 61, e202205894 |
| [16] | Jiang, Y.; Zhang, Z.; Li, S. H.; Cui, D. M. Angew. Chem., Int. Ed. 2022, 61, e202112966. |
| [17] | Ji, G.; Chen, Z.; Wang, X. Y.; Ning, X. S.; Xu, C. J.; Zhang, X. M.; Tao, W. J.; Li, J. F.; Gao, Y. S.; Shen, Q.; Sun, X. L.; Wang, H. Y.; Zhao, J. B.; Zhang, B.; Guo, Y. L.; Zhao, Y. N.; Sun, J. J.; Luo, Y.; Tang, Y. Nat. Commun. 2021, 12, 1. |
| [18] | Tran, T. V.; Do, L. H. Eur. Polym. J. 2021, 142, 110100. |
| [19] | Johnson, L. K.; Killian, C. M.; Brookhart, M. J. Am. Chem. Soc. 1995, 117, 6414. |
| [20] | Johnson, L. K.; Mecking, S.; Brookhart, M. J. Am. Chem. Soc. 1996, 118, 267. |
| [21] | Fischer, K.; Jones, K.; Misbach, P.; Stabba, R.; Wilke G. Angew. Chem., Int. Ed. 1973, 12, 943. |
| [22] | Zou, C.; Si, G. F.; Chen, C. L. Nat. Commun. 2022, 13, 1954. |
| [23] | Wang, Y. Y.; Hu, X. Q.; Mu, H. L.; Xia, Y.; Chi, Y.; Jian, Z. B. Acta Chim. Sinica 2022, 80, 741. (in Chinese) |
| [23] | (王玉银, 胡小强, 穆红亮, 夏艳, 迟悦, 简忠保, 化学学报, 2022, 80, 741.) |
| [24] | Zhang, H.; Zou, C.; Zhao, H.; Cai, Z. G.; Chen, C. L. Angew. Chem., Int. Ed. 2021, 60, 17446. |
| [25] | Liang, T.; Goudari, S.; Chen, C. L. Nat. Commun. 2020, 11, 372. |
| [26] | Lin, F.; Mecking, S. Angew. Chem., Int. Ed. 2022, 134, e202203923. |
| [27] | Xin, B. S.; Sato, N.; Tanna, A.; Oishi, Y.; Konishi, Y.; Shimizu, F. J. Am. Chem. Soc. 2017, 139, 3611. |
| [28] | Zhang, Y.; Mu, H.; Pan, L.; Wang, X.; Li, Y. ACS Catal. 2018, 8, 5963. |
| [29] | Wang, X.; Zhang, Y.; Wang, F.; Pan, L.; Wang, B.; Li, Y. ACS Catal. 2021, 11, 2902. |
| [30] | Xiong, S.; Shoshani, M. M.; Zhang, X.; Spinney, H. A.; Nett, A. J.; Henderson, B. S.; Miller, T. F.; Agapie, T. J. Am. Chem. Soc. 2021, 143, 6516. |
| [31] | Baur, M.; Lin, F.; Morgen, T. O.; Odenwald, L.; Mecking, S. Science 2021, 374, 604. |
| [32] | Mecking, S.; Schnitte, M. Acc. Chem. Res. 2020, 53, 2738. |
| [33] | Du, W.; Zheng, H.; Li, Y.; Cheung, C. S.; Li, D.; Gao, H.; Deng, H.; Gao, H. Macromolecules 2022, 55, 3096. |
| [34] | Chen, S. Y.; Ren, B. H.; Li, S. H.; Song, Y. H.; Jiao, S.; Zou, C.; Chen, C. L.; Lu, X. B.; Liu, Y. Angew. Chem., Int. Ed. 2022, 61, e202204126. |
| [35] | Chen, Z.; Leatherman, M. D.; Daugulis, O.; Brookhart, M. J. Am. Chem. Soc. 2017, 139, 16013. |
| [36] | Chen, Z.; Brookhart, M. Acc. Chem. Res. 2018, 51, 1831. |
| [37] | Guo, L. H.; Dai, S. Y.; Sui, X. L.; Chen, C. L. ACS Catal. 2016, 6, 428. |
| [38] | Wang, F. Z.; Chen, C. L. Polym. Chem. 2019, 10, 2354. |
| [39] | Meinhard, D.; Wegner, M.; Kipiani, G.; Hearley, A.; Reuter, P.; Fischer, S.; Marti O.; Rieger, B. J. Am. Chem. Soc. 2007, 129, 9182. |
| [40] | Vaidya, T.; Klimovica, K.; LaPointe, A. M.; Keresztes, I.; Lobkovsky, E. B.; Daugulis, O.; Coates, G. W. J. Am. Chem. Soc. 2014, 136, 7213. |
| [41] | Wang, Z.; Liu, Q.; Solan, G. A.; Sun, W. H. Coord. Chem. Rev. 2017, 350, 68. |
| [42] | Li, J.; Peng, D.; Tan, C.; Chen, C. L. Angew. Chem., Int. Ed. 2023, 62, e202300359. |
| [43] | Tan, C.; Zou, C.; Chen, C. L. J. Am. Chem. Soc. 2022, 144, 2245. |
| [44] | Anderson Jr, W. C.; Rhinehart, J. L.; Tennyson, A. G.; Long, B. K. J. Am. Chem. Soc. 2016, 138, 774. |
| [45] | Doerr, A. M.; Curry, M. R.; Chapleski, R. C.; Burroughs, J. M.; Lander, E. K.; Roy, S.; Long, B. K. ACS Catal. 2021, 12, 73. |
| [46] | Long, B. K.; Eagan, J. M.; Coates, G. W. S. Angew. Chem., Int. Ed. 2016, 55, 7106. |
| [47] | Zhong, L.; Li, G.; Liang, G.; Gao, H.; Wu, Q. Macromolecules 2017, 50, 2675. |
| [48] | Kanai, Y.; Foro, S.; Plenio, H. Organometallics 2019, 38, 544. |
| [49] | Li, M.; Wang, X. B.; Luo, Y.; Chen, C. L. Angew. Chem., Int. Ed. 2017, 56, 11604. |
| [50] | Peng, D.; Chen, C. L. Angew. Chem., Int. Ed. 2021, 60, 22195. |
| [51] | Liao, Y. D.; Zhang, Y. X.; Cui, L.; Mu, H. L.; Jian, Z. B. Organometallics 2019, 38, 2075. |
| [52] | Pei, L.; Liu, F.; Liao, H.; Gao, J.; Zhong, L.; Gao, H.; Wu, Q. ACS Catal. 2018, 8, 1104. |
| [53] | Hu, X.; Zhang, Y.; Zhang, Y.; Jian, Z. ChemCatChem 2020, 12, 2497. |
| [54] | Gong, Y. F.; Li, S. K.; Gong, Q.; Zhang, S. J.; Liu, B. Y.; Dai, S. Y. Organometallics 2019, 38, 2919. |
| [55] | Fang, J.; Sui, X.; Li, Y.; Chen, C. Polym. Chem. 2018, 9, 4143. |
| [56] | Lu, Z.; Xu, X. W.; Luo, Y.; He, S. B.; Fan, W. G.; Dai, S. Y. ACS Catal. 2023, 13, 725. |
| [57] | Sun, Y.; Wang, Q.; Pan, Y.; Pang, W. M.; Zou, C.; Chen, M. Chin. J. Chem. 2022, 40, 2773. |
| [58] | Mahmood, Q.; Zeng, Y. N.; Yue, E. L.; Solan, G. A.; Liang, T. L.; Sun, W. H. Polym. Chem. 2017, 8, 6416. |
| [59] | Wang, X. X.; Fan, L. L.; Ma, Y. P.; Guo, C. Y.; Solan, G. A.; Sun, Y.; Sun, W. H. Polym. Chem. 2017, 8, 2785. |
| [60] | Chen, A.; Liao, D. H.; Chen, C. L. Chin. J. Chem. 2022, 40, 215. |
| [61] | Peng, D.; Xu, M. H.; Tan, C.; Chen, C. L. Macromolecules 2023, 56, 2388. |
| [62] | Ni1 (CCDC, 2257873); Ni3 (CCDC, 2257874). |
| [63] | Falivene, L.; Credendino, R.; Poater, A.; Petta, A.; Serra, L.; Oliva, R.; Scarano, V.; Cavallo, L. Organometallics 2016, 35, 2286. |
/
| 〈 |
|
〉 |