

串联炔-异氰[3+2]环加成/Boulton-Katritzky重排/扩环反应构建吡咯并[3,2-d]嘧啶-4-酮化合物★
收稿日期: 2023-04-26
网络出版日期: 2023-06-07
基金资助
项目受国家自然科学基金(22001093); 项目受国家自然科学基金(21971087); 广东省基础与应用基础研究项目(2022A1515010200)
Synthesis of Pyrrolo[3,2-d]pyrimidin-4-ones via Cascade Alkyne−isocyanide [3+2] Cycloaddition/Boulton-Katritzky Rearrangement/Ring Expansion Process★
Received date: 2023-04-26
Online published: 2023-06-07
Supported by
National Natural Science Foundation of China(22001093); National Natural Science Foundation of China(21971087); Guangdong Basic and Applied Basic Research Foundation(2022A1515010200)
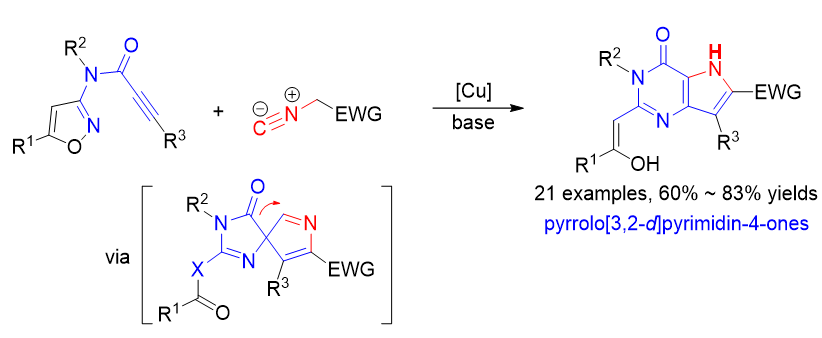
吡咯并[3,2-d]嘧啶-4-酮骨架在药物设计中广受关注, 该骨架是众多生物活性分子的核心结构单元. 目前已报道的吡咯并[3,2-d]嘧啶-4-酮骨架的合成方法有限且存在原料不易得、反应条件苛刻、合成步骤长、产率低和产物结构不易修饰等缺陷. 因此, 发展吡咯并[3,2-d]嘧啶-4-酮骨架的新型高效合成策略在合成化学与药物化学领域均具有重要的研究意义. 最近, 一种通过串联炔-异氰[3+2]环加成/Boulton-Katritzky重排/酰基迁移扩环反应构建9-去氮鸟嘌呤化合物的新方法被报道, 该方法以氮杂异噁唑衍生的炔丙酰胺化合物和异氰化合物为原料. 鉴于异噁唑化合物也是Boulton-Katritzky重排反应常使用的底物, 此工作进一步研究了一系列异噁唑衍生的炔丙酰胺化合物与异氰化合物的上述串联反应, 成功构建了一系列吡咯并[3,2-d]嘧啶-4-酮化合物.
关键词: 炔-异氰[3+2]环加成; Boulton-Katritzky重排; 扩环反应; [3,2-d]嘧啶-4-酮; 串联反应
罗江浩 , 马浩文 , 张杰豪 , 周伟 , 蔡倩 . 串联炔-异氰[3+2]环加成/Boulton-Katritzky重排/扩环反应构建吡咯并[3,2-d]嘧啶-4-酮化合物★[J]. 化学学报, 2023 , 81(8) : 898 -904 . DOI: 10.6023/A23040164
Pyrrolo[3,2-d]pyrimidin-4-ones are valuable structural motifs in many bioactive compounds and pharmaceuticals. Such structures have received extensive attentions from synthetic community. Two general strategies have been developed for the formation of the fused pyrimidine-pyrrole structure: one is to construct the pyrimidine ring from substituted pyrroles, and the other is to construct the pyrrole ring from 5,6-functionalized pyrimidines. However, these methods have generally required multiple synthetic steps and the use of starting materials with uncommon functional groups, and also suffered with other drawbacks such as harsh reaction conditions and limited substrate scope. Thus, it is highly desirable to develop facile and practical approaches for the construction of structural diversified pyrrolo[3,2-d]pyrimidin-4-ones. Very recently, we have developed an alkyne-isocyanide [3+2] cycloaddition/Boulton-Katritzky rearrangement/ring expansion reaction for the synthesis of 9-deazaguanines from 1,2,4-oxadiazole-derived propiolamides with isocyanides. Different from traditional Boulton-Katritzky rearrangement (BKR), which is to form stable five-membered rings, the method provides a facile access to fused heterocycles via forming an unstable BKR spirocyclic intermediate and followed by a spontaneous ring expansion via acyl migration. In this work, to further expand the scope of this method, the [3+2] cycloaddition/BKR-ring expansion reactions of isoxazole-derived propiolamides with isocyanides were developed. The reactions were performed with CuI as the catalyst and Cs2CO3 as the base in toluene/N,N-dimethylformamide (DMF) (1∶3, V∶V) at room temperature for the alkyne-isocyanide [3+2] cycloaddition and then at 110 ℃ for the BKR-ring expansion process. A variety of isoxazole- derived propiolamides and isocyanides were well tolerated in the reactions and afforded the desired pyrrolo[3,2- d]pyrimidin-4-one products in satisfactory yields. The control experiments were performed to elucidate the reaction process: copper catalyst is used only for the alkyne-isocyanide [3+2] cycloaddition, but no necessary for the base-promoted BKR-ring expansion process. Compared to the traditional methods for such skeletons, the approach features readily available starting materials, broad substrate scope, short steps, and structural diversification.

| [1] | (a) Bantia S.; Miller P. J.; Parker C. D.; Ananth S. L.; Horn L. L.; Kilpatrick J. M.; Morris P. E.; Hutchison T. L.; Montgomery J. A.; Sandhu J. S.; Int. Immunopharmacol. 2001, 1, 1199; |
| [1] | (b) Theoclitou M.-E.; Aquila, B.; Block, M. H.; Brassil, P. J.; Castriotta, L.; Code, E.; Collins, M. P.; Davies, A. M.; Deegan, T.; Ezhuthachan, J.; Filla, S.; Freed, E.; Hu, H.; Huszar, D.; Jayaraman, M.; Lawson, D.; Lewis, P. M.; Nadella, M. V. P.; Oza, V.; Padmanilayam, M.; Pontz, T.; Ronco, L.; Russell, D.; Whitston, D.; Zheng, X. J. Med. Chem. 2011, 54, 6734; |
| [1] | (c) Laufersweiler M. C.; Wang Y.; Soper D. L.; Suchanek M. K.; Fancher A. N.; Lu W.; Wang R. L.; Oppong K. A.; Ellis C. D.; Baize M. W.; O'Neil S. V.; Wos J. A.; Demuth T. P. Bioorg. Med. Chem. Lett. 2005, 15, 4322. |
| [2] | (a) Semeraro T.; Lossani A.; Botta M.; Ghiron C.; Alvarez R.; Manetti F.; Mugnaini C.; Valensin S.; Focher F.; Corelli F.; J. Med. Chem. 2006, 49, 6037; |
| [2] | (b) Rodrigues M. V. N.; Barbosa, A. F.; da Silva, J. F.; dos Santos, D. A.; Vanzolini, K. L.; de Moraes, M. C.; Corrêa, A. G.; Cass, Q. B. Bioorg. Med. Chem. 2016, 24, 226; |
| [2] | (c) Tian C.; Wang, M.; Fang, F.; Zhang, Z.; Wang, X.; Liu, J. Eur. J. Med. Chem. 2017, 138, 630; |
| [2] | (d) Gangjee A.; Li, W.; Yang, J.; Kisliuk, R. L. J. Med. Chem. 2008, 51, 68; |
| [2] | (e) Huang L.; Li, H.; Li, L.; Niu, L.; Seupel, R.; Wu, C.; Cheng, W.; Chen, C.; Ding, B.; Brennan, P. E.; Yang, S. J. Med. Chem. 2019, 62, 4526; |
| [2] | (f) Zeng S.; Xie H.; Zeng L.-L.; Lu X.; Zhao X.; Zhang G.-C.; Tu Z.-C.; Xu H.-J.; Yang L.; Zhang X.-Q.; Hu W. Bioorg. Med. Chem. 2013, 21, 1749. |
| [3] | (a) Imai K.-I. Chem. Pharm. Bull. 1964, 12, 1030 |
| [3] | (b) Taylor, E. C.; Young, W. B.; Ward, C. C. Tetrahedron Lett. 1993, 34, 4595;. |
| [3] | (c) Taylor, E. C.; Young, W. B. J. Org. Chem. 1995, 60, 7947;. |
| [3] | (d) Elliott, A. J.; Montgomery, J. A.; Walsh, D. A. Tetrahedron Lett. 1996, 37, 4339;. |
| [3] | (e) Furneaux, R. H.; Tyler, P. C. J. Org. Chem. 1999, 64, 8411;. |
| [3] | (f) Liu, M.-C.; Luo, M.-Z.; Mozdziesz, D. E.; Sartorelli, A. C. Synth. Commun. 2002, 32, 3797;. |
| [3] | (g) Elliott, A. J.; Morris, P. E.; Petty, Jr. S. L.; Williams, C. H. J. Org. Chem. 1997, 62, 8071;. |
| [3] | (h) Kwong, C. D.; Elliot, A. J.; Montgomery, J. A. J. Labelled Cpd. Radiopharm. 1998, 41, 879;. |
| [3] | (i) Shih H.; Cottam H. B.; Carson D. A. Chem. Pharm. Bull. 2002, 50, 364. |
| [4] | (a) Boulton A. J.; Katritzky A. R.; Hamid A. M. J. Chem. Soc. C 1967, 2005; |
| [4] | (b) Afridi, A. S.; Katritzky, A. R.; Ramsden, C. A. J. Chem. Soc., Perkin Trans. 1 1976, 315;. |
| [4] | (c) For a book: Comprehensive Heterocyclic Chemistry II, Eds.: Katritzky, A. R.; Rees, C. W.; Scriven, E. F. V., Elsevier, Amsterdam, 1996, Vols. 1-9. |
| [5] | For selected reviews and examples of rearrangement with isoxazoles and 1,2,4-oxadiazoles, see: (a) Pace, A.; Pierro, P.; Buscemi, S.; Vivona, N.; Barone, G. J. Org. Chem. 2009, 74, 351; |
| [5] | (b) Martorana, A.; Piccionello, A. P.; Buscemi, S.; Giorgi, G.; Pace, A. Org. Biomol. Chem. 2011, 9, 491;. |
| [5] | (c) Martorana, A.; Pace, A.; Buscemi, S.; Piccionello, A. P. Org. Lett. 2012, 14, 3240;. |
| [5] | (d) Hu, F.; Szostak, M. Adv. Synth. Catal. 2015, 357, 2583;. |
| [5] | (e) Jones R. C. F.; Chatterley A.; Marty R.; Owton W. M.; Elsegood M. R. J. Chem. Commun. 2015, 51, 1112. |
| [6] | For selected reviews on ring expansion reactions, see: (a) Lu, B.-L.; Dai, L.; Shi, M. Chem. Soc. Rev. 2012, 41, 3318; |
| [6] | (b) Mack, D. J.; Njardarson, J. T. ACS Catal. 2013, 3, 272; |
| [6] | (c) Biletskyi, B.; Colonna, P.; Masson, K.; Parrain, J.-L.; Commeiras, L.; Chouraqui, G. Chem. Soc. Rev. 2021, 50, 7513; |
| [6] | (d) Li, D.; Zang, W.; Bird, M. J.; Hyland, C. J. T.; Shi, M. Chem. Rev. 2021, 121, 8685; |
| [6] | (e) Nanda, T.; Fastheem, M.; Linda, A.; Pati, B. V.; Banjare, S. K.; Biswal, P.; Ravikumar, P. C. ACS Catal. 2022, 12, 13247; |
| [6] | (f) Ye J. Chin. J. Org. Chem. 2021, 41, 1755. |
| [7] | For selected examples on ring expansion reactions, see: (a) Luzung, M. R.; Markham, J. P.; Toste, F. D. J. Am. Chem. Soc. 2004, 126, 10858; |
| [7] | (b) Gorin, D. J.; Davis, N. R.; Toste, F. D. J. Am. Chem. Soc. 2005, 127, 11260; |
| [7] | (c) Chen, G.-Q.; Zhang, X. N.; Wei, Y.; Tang, X.-Y.; Shi, M. Angew. Chem., Int. Ed. 2014, 53, 8492; |
| [7] | (d) Pan, D.; Wei, Y.; Shi, M. Org. Lett. 2016, 18, 3930; |
| [7] | (e) Chen, G.-Q.; Fang, W.; Wei, Y.; Tang, X.-Y.; Shi, M. Chem. Sci. 2016, 7, 4318; |
| [7] | (f) Pan, D.; Wei, Y.; Shi, M. Org. Lett. 2017, 19, 3584; |
| [7] | (g) Wu, Q.-F.; Zheng, C.; You, S.-L. Angew. Chem., Int. Ed. 2012, 51, 1680; |
| [7] | (h) Zhuo, C.-X.; Wu, Q.-F.; Zhao, Q.; Xu, Q.-L.; You, S.-L. J. Am. Chem. Soc. 2013, 135, 8169; |
| [7] | (i) Zhuo, C.-X.; Cheng, Q.; Liu, W.-B.; Zhao, Q.; You, S.-L. Angew. Chem., Int. Ed. 2015, 54, 8475; |
| [7] | (j) Wu, Q.; Zheng, C.; Zhuo, C.-X.; You, S.-L. Chem. Sci. 2016, 7, 4453; |
| [7] | (k) Wang, Y.; Zheng, C.; You, S.-L. Angew. Chem., Int. Ed. 2017, 56, 15093; |
| [7] | (l) Zheng, C.; You, S.-L. Acc. Chem. Res. 2020, 53, 974; |
| [7] | (m) George, J.; Kim, H. Y.; Oh, K. Adv. Synth. Catal. 2016, 358, 3714; |
| [7] | (n) George, J.; Kim, H. Y.; Oh, K. Org. Lett. 2017, 19, 628; |
| [7] | (o) Ramu, G.; Ambala, S.; Nanubolu, J. B.; Babu, B. N. RSC Adv. 2019, 9, 35068; |
| [7] | (p) Ramu, G.; Tangella, Y.; Ambala, S.; Babu, B. N. J. Org. Chem. 2020, 85, 5370; |
| [7] | (q) Moisan, L.; Wagner, M.; Comesse, S.; Doris, E. Tetrahedron Lett. 2006, 47, 9093; |
| [7] | (r) Pesquet, A.; Da?ch, L. Van Hijfte, J. Org. Chem. 2006, 71, 5303; |
| [7] | (s) Shang, S.; Zhang-Negrerie, D.; Du, Y.; Zhao, K. Angew. Chem., Int. Ed. 2014, 53, 6216; |
| [7] | (t) Guo, X.; Xing, Q.; Lei, K.; Zhang-Negrerie, D.; Du, Y.; Zhao, K. Adv. Synth. Catal. 2017, 359, 4393; |
| [7] | (u) Mamedov, V. A.; Mamedova, V. L.; Qu, Z.-W.; Zhu, H.; Galimullina, V. R.; Korshin, D. E.; Khikmatova, G. Z.; Litvinov, I. A.; Latypov, S. K.; Sinyashin, O. G.; Grimme, S. J. Org. Chem. 2021, 86, 13514; |
| [7] | (v) Mandal, S.; Pramanik, A. J. Org. Chem. 2022, 87, 9282; |
| [7] | (w) Zhu W.-K.; Xu L.-W. Chin. J. Org. Chem. 2023, 43, 362. (in Chinese) |
| [7] | ( 祝炜柯, 徐利文, 有机化学, 2023, 43, 362.) |
| [8] | For recent reviews on heterocyclic dearomatization: (a) Roche, S. P.; Porco, J. A. Angew. Chem., Int. Ed. 2011, 50, 4068; |
| [8] | (b) Donohoe, T. J.; Pullin, R. D. C. Chem. Commun. 2012, 48, 11924; |
| [8] | (c) Ding, Q.; Zhou, X.; Fan, R. Org. Biomol. Chem. 2014, 12, 4807; |
| [8] | (d) Zhuo, C.-X.; Zheng, C.; You, S.-L. Acc.Chem. Res. 2014, 47, 2558; |
| [8] | (e) Liang, X.-W.; Zheng, C.; You, S.-L. Chem. Eur. J. 2016, 22, 11918; |
| [8] | (f) Sun, W.; Li, G.; Hong, L.; Wang, R. Org. Biomol. Chem. 2016, 14, 2164; |
| [8] | (g) Zheng, C.; You, S.-L. Chem 2016, 1, 830; |
| [8] | (h) Wu W.-T.; Zhang L.-M.; You S.-L. Acta Chim. Sinica 2017, 75, 419. (in Chinese) |
| [8] | ( 吴文挺, 张立明, 游书力, 化学学报, 2017, 75, 419.) |
| [9] | Luo J.; Ma H.; Wu K.; Ao Y.; Zhou W.; Cai Q. Org. Lett. 2023, 25, 2123. |
| [10] | For selected reviews on alkyne-isocyanide [3+2] cycloaddition, see: (a) Gulevich, A. V.; Zhdanko, A. G.; Orru, R. V. A.; Nenajdenko, V. G. Chem. Rev. 2010, 110, 5235; |
| [10] | (b) Boyarskiy, V. P.; Bokach, N. A.; Luzyanin, K. V.; Kukushkin, V. Y. Chem. Rev. 2015, 115, 2698; |
| [10] | (c) Giustiniano, M.; Basso, A.; Mercalli, V.; Massarotti, A.; Novellino, E.; Tron, G. C.; Zhu, J. Chem. Soc. Rev. 2017, 46, 1295; |
| [10] | (d) Luo J.; Chen G.-S.; Chen S.-J.; Li Z.-D.; Liu Y.-L. Chem. Eur. J. 2021, 27, 6598. |
| [11] | For selected examples, see: (a) Kamijo, S.; Kanazawa, C.; Yamamoto, Y. J. Am. Chem. Soc. 2005, 127, 9260; |
| [11] | (b) Larionov, O. V.; de Meijere, A. Angew. Chem., Int. Ed. 2005, 44, 5664; |
| [11] | (c) Gao, M.; He, C.; Chen, H.; Bai, R.; Cheng, B.; Lei, A. Angew. Chem., Int. Ed. 2013, 52, 6958; |
| [11] | (d) Liu, J.; Fang, Z.; Zhang, Q.; Liu, Q.; Bi, X. Angew. Chem., Int. Ed. 2013, 52, 6953; |
| [11] | (e) Qi, X.; Zhang, H.; Shao, A.; Zhu, L.; Xu, T.; Gao, M.; Liu, C.; Lan, Y. ACS Catal. 2015, 5, 6640; |
| [11] | (f) Xiao, P.; Yuan, H.; Liu, J.; Zheng, Y.; Bi, X.; Zhang, J. ACS Catal. 2015, 5, 6177; |
| [11] | (g) Dong, J.; Bao, L.; Hu, Z.; Ma, S.; Zhou, X.; Hao, M.; Li, N.; Xu, X. Org. Lett. 2018, 20, 1244; |
| [11] | (h) He, X.-L.; Zhao, H.-R.; Song, X.; Jiang, B.; Du, W.; Chen, Y.-C.; ACS Catal. 2019, 9, 4374; |
| [11] | (i) Zheng, S.-C.; Wang, Q.; Zhu, J. Angew. Chem., Int. Ed. 2019, 58, 1494; |
| [11] | (j) Liu, J.-Q.; Chen, X.; Shatskiy, A.; K?rk?s, M. D.; Wang, X.-S. J. Org. Chem. 2019, 84, 8998; |
| [11] | (k) Wang Y.; Zhou Y.; Song Q. Chem. Commun. 2020, 56, 6106. |
| [12] | Bird C. W. Tetrahedron 1985, 41, 1409. |
| [13] | CCDC 2258385 (3j) contains the supplementary crystallographic data for this paper. |
/
| 〈 |
|
〉 |