

团簇Au/CeO2的制备及其催化CO氧化反应构效关系的研究★
收稿日期: 2023-04-28
网络出版日期: 2023-06-12
基金资助
项目受国家重点研发项目(2021YFA1501103); 国家杰出青年基金项目(22225110); 国家自然科学基金(22075166); 国家自然科学基金(22271177); 山东大学未来学者项目资助
Fabrication and Mechanism Study of Clustered Au/CeO2 Catalyst for the CO Oxidation Reaction★
Received date: 2023-04-28
Online published: 2023-06-12
Supported by
National Key Research and Development Program of China(2021YFA1501103); National Science Fund for Distinguished Young Scholars of China(22225110); National Natural Science Foundation of China(22075166); National Natural Science Foundation of China(22271177); Young Scholars Program of Shandong University
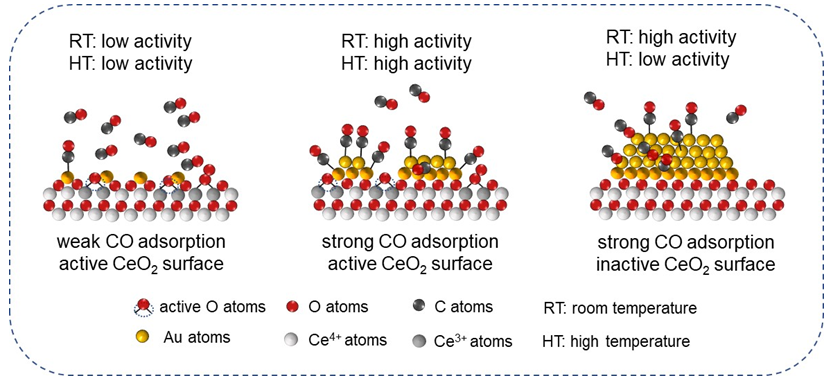
负载型Au催化剂因其在诸多反应过程中的高催化活性而备受研究者关注. 然而针对负载型催化剂中Au物种结构的有效调控, 以及催化过程中真实构-效关系的探索一直充满了挑战. 用CeO2为Au物种担载基底, 通过简单煅烧处理引起的CeO2结构变化, 进而实现Au/CeO2之间界面作用力的调控. 此研究发现Au纳米颗粒中Au0物种具备更为高效的催化室温CO氧化活性, 结合多种原位表征分析, 其室温条件下催化转化效率更依赖于CO吸附能力. 而相比于单原子Au1和纳米Au颗粒, 所制备的团簇Au/CeO2催化剂在较高温度(>50 ℃)展现出优异的催化CO氧化反应性能. 随着温度升高, 催化剂表界面O参与的MvK反应路径更易发生, 因此具有更多表界面活性O物种和Auδ+位点的团簇Au/CeO2催化剂展现出最为优异的催化CO氧化性能. 这些发现为高效负载型Au催化剂的制备提供了新思路并深化了对Au/CeO2催化作用机制的理解.
付信朴 , 王秀玲 , 王伟伟 , 司锐 , 贾春江 . 团簇Au/CeO2的制备及其催化CO氧化反应构效关系的研究★[J]. 化学学报, 2023 , 81(8) : 874 -883 . DOI: 10.6023/A23040174
Supported Au-based catalysts have been attracting continuous attention owing to their outstanding performance in various catalytic applications. However, the complicated environment on the catalyst surface severely hampered the unambiguous illustration of the structural-function relationship for Au-based catalysts. In this work, we developed a facile strategy to fabricate various Au species [Aun+ (n>1), Auδ+ (0<n<1) and Au0] onto the CeO2 support, which the Au/CeO2 interaction was distinctively modulated by the CeO2-x with different calcination temperatures. As-prepared Au/CeO2-x were valued as catalysts for CO oxidation reaction, which is important for both environmental application and model catalysis. The as-formed clustered Au with δ+ oxidation state on CeO2-400 demonstrates the best catalytic performance at 50 ℃, while the Au nanoparticles with dominant Au0 atoms are superior for catalyzing CO oxidation at room temperature. However, the monodispersed Aun+ single-sites with the highest dispersion are almost inactive for CO oxidation below 50 ℃. On the basis of structural characterizations and in-situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) results, we reasonably speculated that the electronic state of Au species plays a predominant role at room temperature for catalytic performance owing to the differentiated CO adsorption ability. This speculation is well in line with our former research finding that the Aun+ sites are weak in capturing CO molecules and Au0 is favorable for CO adsorption. The surficial O atoms, especially the lattice O atoms, play a minor role in catalyzing CO oxidation for the Au particles within Au/CeO2-700, implying that the reactant molecules might prefer a L-H pathway at room temperature. In contrast, the clustered Auδ+ with moderate CO adsorption ability and abundant interfacial site was apt to participate in the surface reaction with a MvK pathway, in which the reaction between surficial lattice O atoms and adsorbed CO molecules significantly contribute to the catalytic activity. Therefore, the Au/CeO2-400 catalyst coupling with moderate CO adsorption ability and abundant active O atoms displayed the best catalytic efficiency at elevated temperatures. These findings in this work provided a facile route for the fabrication efficient Au/CeO2 catalyst, and shed light on the molecular understanding of the reaction path over various Au sites.

| [1] | Haruta M.; Kobayashi T.; Sano H.; Yamada N. Chem. Lett. 1987, 16, 405. |
| [2] | Hutchings G. J. J. Catal. 1985, 96, 292. |
| [3] | Jin Z.; Song Y. Y.; Fu X. P.; Song Q. S.; Jia C. J. Chin. J. Chem. 2018, 36, 639. |
| [4] | Camellone M. F.; Fabris S. J. Am. Chem. Soc. 2009, 131, 10473. |
| [5] | Saavedra J.; Doan H. A.; Pursell C. J.; Grabow L. C.; Chandler B. D. Science 2014, 345, 1599. |
| [6] | Liu L.; Corma A. Chem. Rev. 2018, 118, 4981. |
| [7] | Ha H.; Yoon S.; An K.; Kim H. Y. ACS Catal. 2018, 8, 11491. |
| [8] | Lee Y.; He G.; Akey A. J.; Si R.; Flytzani-Stephanopoulos M.; Herman I. P. J. Am. Chem. Soc. 2011, 133, 12952. |
| [9] | Boccuzzi F.; Chiorino A.; Manzoli M.; Lu P.; Akita T.; Ichikawa S.; Haruta M. J. Catal. 2001, 202, 256. |
| [10] | Guo L.-W.; Du P.-P.; Fu X.-P.; Ma C.; Zeng J.; Si R.; Huang Y.-Y.; Jia C.-J.; Zhang Y.-W.; Yan C.-H. Nat. Commun. 2016, 7, 1. |
| [11] | Schlexer P.; Widmann D.; Behm R. J.; Pacchioni G. ACS Catal. 2018, 8, 6513. |
| [12] | Rodriguez J. A.; Grinter D. C.; Liu Z.; Palomino R. M.; Senanayake S. D. Chem. Soc. Rev. 2017, 46, 1824. |
| [13] | Ke J.; Zhu W.; Jiang Y.; Si R.; Wang Y.-J.; Li S.-C.; Jin C.; Liu H.; Song W.-G.; Yan C.-H.; Zhang Y.-W. ACS Catal. 2015, 5, 5164. |
| [14] | Teng B.-C.; Jiang S.-Y.; Guo X.-W.; Yuan J.-H.; Luo M.-F. Acta Chim. Sinica 2009, 67, 2765. (in Chinese) |
| [14] | ( 滕波涛, 蒋仕宇, 郭晓伟, 袁金焕, 罗孟飞, 化学学报, 2009, 67, 2765.) |
| [15] | Jiao T.; Xu X.-L.; Zhang L.; Weng Y.-Y.; Weng Y.-B.; Gao Z.-X. Acta Chim. Sinica 2021, 79, 513. (in Chinese) |
| [15] | ( 焦桐, 许雪莲, 张磊, 翁幼云, 翁玉冰, 高志贤, 化学学报, 2021, 79, 513.) |
| [16] | Lin J.; Zhang L.; Ni J.; Wang R.; Wei K. Acta Chim. Sinica 2012, 70, 137. (in Chinese) |
| [16] | ( 林建新, 张留明, 倪军, 王榕, 魏可镁, 化学学报, 2012, 70, 137.) |
| [17] | Spezzati G.; Benavidez A. D.; DeLaRiva A. T.; Su Y.; Hofmann J. P.; Asahina S.; Olivier E. J.; Neethling J. H.; Miller J. T.; Datye A. K.; Hensen E. J. M. Appl. Catal. B: Environ. 2019, 243, 36. |
| [18] | Sun X.-C.; Yuan K.; Hua W.-D.; Gao Z.-R.; Zhang Q.; Yuan C.-Y.; Liu H.-C.; Zhang Y.-W. ACS Catal. 2022, 12, 11942. |
| [19] | Si R.; Flytzani-Stephanopoulos M. Angew. Chem. Int. Ed. 2008, 47, 2884. |
| [20] | Zhang S.; Lee J.; Kim D. H.; Kim T. Molecular Catalysis 2020, 482. |
| [21] | Yu W.-Z.; Wang W.-W.; Li S.-Q.; Fu X.-P.; Wang X.; Wu K.; Si R.; Ma C.; Jia C.-J.; Yan C.-H. J. Am. Chem. Soc. 2019, 141, 17548. |
| [22] | Manibalan G.; Murugadoss G.; Thangamuthu R.; Kumar R. M.; Jayavel R.; Kumar M. R. Materials Research Express 2019, 6, 075032. |
| [23] | Mihaylov M.; Kn?zinger H.; Hadjiivanov K.; Gates B. C. Chem. Ing. Tech. 2007, 79, 795. |
| [24] | McEntee M.; Stevanovic A.; Tang W.; Neurock M.; Yates J. T. Jr. J. Am. Chem. Soc. 2015, 137, 1972. |
| [25] | Zhang S.; Li X.-S.; Chen B.; Zhu X.; Shi C.; Zhu A.-M. ACS Catal. 2014, 4, 3481. |
| [26] | El-Moemen A. A.; Ku?erová G.; Behm R. J. Appl. Catal. B: Environ. 2010, 95, 57. |
| [27] | Kang L.; Wang B.; Bing Q.; Zalibera M.; Buchel R.; Xu R.; Wang Q.; Liu Y.; Gianolio D.; Tang C. C.; Gibson E. K.; Danaie M.; Allen C.; Wu K.; Marlow S.; Sun L. D.; He Q.; Guan S.; Savitsky A.; Velasco-Velez J. J.; Callison J.; Kay C. W. M.; Pratsinis S. E.; Lubitz W.; Liu J. Y.; Wang F. R. Nat. Commun. 2020, 11, 4008. |
| [28] | Kim H. Y.; Lee H. M.; Henkelman G. J. Am. Chem. Soc. 2012, 134, 1560. |
/
| 〈 |
|
〉 |