

铜催化的二醇类化合物对映选择性去对称化反应研究进展★
收稿日期: 2023-04-23
网络出版日期: 2023-06-13
基金资助
项目受国家自然科学基金(22025103); 项目受国家自然科学基金(92256301); 项目受国家自然科学基金(21831002); 项目受国家自然科学基金(22101122); 项目受国家自然科学基金(22001109); 项目受国家自然科学基金(22271133); 项目受国家自然科学基金(22201127); 国家重点研发计划(2021YFF0701604); 国家重点研发计划(2021YFF0701704); 深圳市科技计划(KQTD20210811090112004); 深圳市科技计划(JCYJ20220530115409020)
Research Progress on Copper-Catalyzed Enantioselective Desymmetrization of Diols★
Received date: 2023-04-23
Online published: 2023-06-13
Supported by
National Natural Science Foundation of China(22025103); National Natural Science Foundation of China(92256301); National Natural Science Foundation of China(21831002); National Natural Science Foundation of China(22101122); National Natural Science Foundation of China(22001109); National Natural Science Foundation of China(22271133); National Natural Science Foundation of China(22201127); National Key R&D Program of China(2021YFF0701604); National Key R&D Program of China(2021YFF0701704); Shenzhen Science and Technology Program(KQTD20210811090112004); Shenzhen Science and Technology Program(JCYJ20220530115409020)
鱼章龙 , 李忠良 , 杨昌江 , 顾强帅 , 刘心元 . 铜催化的二醇类化合物对映选择性去对称化反应研究进展★[J]. 化学学报, 2023 , 81(8) : 955 -966 . DOI: 10.6023/A23040161
Enantioselective desymmetrization of diols is an important method for the synthesis of complex enantioenriched alcohols, which has broad application prospects in medicinal chemistry, total synthesis, and materials science. In recent years, the use of copper catalysis to achieve diols’ enantioselective desymmetrization has progressed rapidly because copper is inexpensive and readily available compared with other noble metal catalysts. Besides, copper undergoes a two-electron or single-electron transfer process in the catalytic cycle, and the rich oxidation states of copper provides the opportunity to solve some challenging problems. This review summarizes the research progress in this field according to the types of diols (meso diol and prochiral diol) and reactions together with a brief perspective.
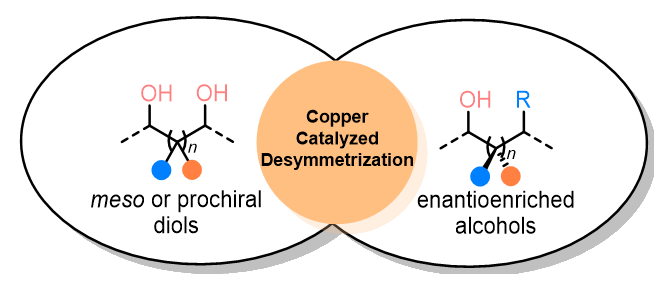
Key words: copper catalysis; desymmetrization; diols; asymmetric catalysis
| [1] | (a) Borissov A.; Davies T. Q.; Ellis S. R.; Fleming T. A.; Richardson M. S.; Dixon D. J. Chem. Soc. Rev. 2016, 45, 5474. |
| [1] | (b) Petersen K. S. Tetrahedron Lett. 2015, 56, 6523. |
| [1] | (c) Nájera C.; Foubelo F.; Sansano J. M.; Yus M. Tetrahedron 2022, 106-107, 132629. |
| [1] | (d) Xu Y.; Zhai T.-Y.; Xu Z.; Ye L.-W. Trends in Chemistry 2022, 4, 191. |
| [1] | (e) Teng M.-Y.; Han T.; Huang E.-H.; Ye L.-W. Chin. J. Org. Chem. 2022, 42, 3295. (in Chinese) |
| [1] | ( 滕明瑜, 韩涛, 黄恩和, 叶龙武, 有机化学, 2022, 42, 3295.) |
| [1] | (f) Yang B.; Yang J.; Zhang J. Chin. J. Chem. 2022, 40, 317. |
| [1] | (g) Deng Z.; Ouyang Y.; Ao Y.; Cai Q. Acta Chim. Sinica 2021, 79, 649. (in Chinese) |
| [1] | ( 邓卓基, 欧阳溢凡, 敖运林, 蔡倩, 化学学报, 2021, 79, 649.) |
| [1] | (h) Zhao W.; Liu J.; He X.; Jiang H.; Lu L.; Xiao W. Chin. J. Org. Chem. 2022, 42, 2504. (in Chinese) |
| [1] | ( 赵薇, 刘京, 何向奎, 蒋豪, 陆良秋, 肖文精, 有机化学, 2022, 42, 2504.) |
| [1] | (i) Yuan H.; Wang J. Chin. J. Org. Chem. 2022, 42, 302. (in Chinese) |
| [1] | ( 袁海瑞, 王剑波, 有机化学, 2022, 42, 302.) |
| [2] | Zeng X.-P.; Cao Z.-Y.; Wang Y.-H.; Zhou F.; Zhou J. Chem. Rev. 2016, 116, 7330. |
| [3] | García-Urdiales E.; Alfonso I.; Gotor V. Chem. Rev. 2011, 111, PR110. |
| [4] | (a) Muller C. E.; Schreiner P. R. Angew. Chem., Int. Ed. 2011, 50, 6012. |
| [4] | (b) Enriquez-Garcia A.; Kundig E. P. Chem. Soc. Rev. 2012, 41, 7803. |
| [4] | (c) Díaz-de-Villegas M. D.; Gálvez J. A.; Badorrey R.; López-Ram-de-Viu M. P. Chem. Eur. J. 2012, 18, 13920. |
| [4] | (d) Suzuki T. Tetrahedron Lett. 2017, 58, 4731. |
| [5] | (a) Trost B. M.; Mino T. J. Am. Chem. Soc. 2003, 125, 2410. |
| [5] | (b) Honjo T.; Nakao M.; Sano S.; Shiro M.; Yamaguchi K.; Sei Y.; Nagao Y. Org. Lett. 2007, 9, 509. |
| [5] | (c) Trost B. M.; Malhotra S.; Mino T.; Rajapaksa N. S. Chem. Eur. J. 2008, 14, 7648. |
| [6] | (a) Cao K.-S.; Zheng W.-H. Tetrahedron: Asymmetry 2015, 26, 1150. |
| [6] | (b) Yang W.; Yan J.; Long Y.; Zhang S.; Liu J.; Zeng Y.; Cai Q. Org. Lett. 2013, 15, 6022. |
| [6] | (c) Shi J.; Wang T.; Huang Y.; Zhang X.; Wu Y.-D.; Cai Q. Org. Lett. 2015, 17, 840. |
| [7] | Zhou Q.-Q.; Lu F.-D.; Liu D.; Lu L.-Q.; Xiao W.-J. Org. Chem. Front. 2018, 5, 3098. |
| [8] | (a) Zi W.; Toste F. D. Angew. Chem., Int. Ed. 2015, 54, 14447. |
| [8] | (b) Zheng Y.; Guo L.; Zi W. Org. Lett. 2018, 20, 7039. |
| [9] | Ouellette E. T.; Lougee M. G.; Bucknam A. R.; Endres P. J.; Kim J. Y.; Lynch E. J.; Sisko E. J.; Sculimbrene B. R. J. Org. Chem. 2021, 86, 7450. |
| [10] | Solomon E. I.; Heppner D. E.; Johnston E. M.; Ginsbach J. W.; Cirera J.; Qayyum M.; Kieber-Emmons M. T.; Kjaergaard C. H.; Hadt R. G.; Tian L. Chem. Rev. 2014, 114, 3659. |
| [11] | Matsumura Y.; Maki T.; Murakami S.; Onomura O. J. Am. Chem. Soc. 2003, 125, 2052. |
| [12] | Mazet C.; Kohler V.; Pfaltz A. Angew. Chem., Int. Ed. 2005, 44, 4888. |
| [13] | Nakamura D.; Kakiuchi K.; Koga K.; Shirai R. Org. Lett. 2006, 8, 6139. |
| [14] | Arai T.; Mizukami T.; Yanagisawa A. Org. Lett. 2007, 9, 1145. |
| [15] | Ka?u?a Z.; Bielawski K.; ?wiek R.; Niedziejko P.; Kaliski P. Tetrahedron: Asymmetry 2013, 24, 1435. |
| [16] | Canipa S. J.; Stute A.; O'Brien P. Tetrahedron 2014, 70, 7395. |
| [17] | Matsumoto K.; Mitsuda M.; Ushijima N.; Demizu Y.; Onomura O.; Matsumura Y. Tetrahedron Lett. 2006, 47, 8453. |
| [18] | Hashimoto Y.; Michimuko C.; Yamaguchi K.; Nakajima M.; Sugiura M. J. Org. Chem. 2019, 84, 9313. |
| [19] | Demizu Y.; Matsumoto K.; Onomura O.; Matsumura Y. Tetrahedron Lett. 2007, 48, 7605. |
| [20] | Onomura O.; Arimoto H.; Matsumura Y.; Demizu Y. Tetrahedron Lett. 2007, 48, 8668. |
| [21] | Hamaguchi N.; Kuriyama M.; Onomura O. Tetrahedron: Asymmetry 2016, 27, 177. |
| [22] | Li R. Z.; Tang H.; Yang K. R.; Wan L. Q.; Zhang X.; Liu J.; Fu Z.; Niu D. Angew. Chem., Int. Ed. 2017, 56, 7213. |
| [23] | Mitsuda M.; Tanaka T.; Tanaka T.; Demizu Y.; Onomura O.; Matsumura Y. Tetrahedron Lett. 2006, 47, 8073. |
| [24] | Onomura O.; Demizu Y.; Kubo Y.; Matsumura Y. Synlett 2008, 2008, 433. |
| [25] | Jung B.; Hong M. S.; Kang S. H. Angew. Chem., Int. Ed. 2007, 46, 2616. |
| [26] | Jung B.; Kang S. H. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 1471. |
| [27] | Hong M. S.; Kim T. W.; Jung B.; Kang S. H. Chem. Eur. J. 2008, 14, 3290. |
| [28] | Lee J. Y.; You Y. S.; Kang S. H. J. Am. Chem. Soc. 2011, 133, 1772. |
| [29] | You Y. S.; Kim T. W.; Kang S. H. Chem. Commun. 2013, 49, 9669. |
| [30] | Shimizu M.; Mushika M.; Mizota I.; Zhu Y. RSC Adv. 2019, 9, 23400. |
| [31] | Kuriyama M.; Tanigawa S.; Kubo Y.; Demizu Y.; Onomura O. Tetrahedron: Asymmetry 2010, 21, 1370. |
| [32] | Tsuda Y.; Kuriyama M.; Onomura O. Chem. Eur. J. 2012, 18, 2481. |
| [33] | Yamamoto K.; Tsuda Y.; Kuriyama M.; Demizu Y.; Onomura O. Chem. - Asian J. 2020, 15, 840. |
| [34] | Yamamoto K.; Ishimaru S.; Oyama T.; Tanigawa S.; Kuriyama M.; Onomura O. Org. Process Res. Dev. 2019, 23, 660. |
| [35] | Yamamoto K.; Suganomata Y.; Inoue T.; Kuriyama M.; Demizu Y.; Onomura O. J. Org. Chem. 2022, 87, 6479. |
| [36] | Yang W.; Liu Y.; Zhang S.; Cai Q. Angew. Chem., Int. Ed. 2015, 54, 8805. |
| [37] | Zhang Y.; Wang Q.; Wang T.; He H.; Yang W.; Zhang X.; Cai Q. J. Org. Chem. 2017, 82, 1458. |
| [38] | Wang Q.; Ye F.; Cao J.; Xu Z.; Zheng Z.-J.; Xu L.-W. Catal. Commun. 2020, 138, 105950. |
| [39] | Gao J.; Mai P.-L.; Ge Y.; Yuan W.; Li Y.; He C. ACS Catal. 2022, 12, 8476. |
| [40] | Cheng Y.-F.; Liu J.-R.; Gu Q.-S.; Yu Z.-L.; Wang J.; Li Z.-L.; Bian J.-Q.; Wen H.-T.; Wang X.-J.; Hong X.; Liu X.-Y. Nat. Catal. 2020, 3, 401. |
| [41] | Cheng Y.-F.; Yu Z.-L.; Tian Y.; Liu J.-R.; Wen H.-T.; Jiang N.-C.; Bian J.-Q.; Xu G.-X.; Xu D.-T.; Li Z.-L.; Gu Q.-S.; Hong X.; Liu X.-Y. Nat. Chem. 2023, 15, 395. |
| [42] | Yu Z.-L.; Cheng Y.-F.; Liu J.-R.; Yang W.; Xu D.-T.; Tian Y.; Bian J.-Q.; Li Z.-L.; Fan L.-W.; Luan C.; Gao A.; Gu Q.-S.; Liu X.-Y. J. Am. Chem. Soc. 2023, 145, 6535. |
/
| 〈 |
|
〉 |