

4-单取代-1,2,3-三氮唑的修饰转化研究进展
收稿日期: 2023-06-12
网络出版日期: 2023-08-15
基金资助
国家自然科学基金(21662020); 国家自然科学基金(21901091); 院士(专家)工作站项目云南省王源超专家工作站(202305AF150018)
Advances in the Modifications of 4-Monosubstituted 1,2,3-Triazoles
Received date: 2023-06-12
Online published: 2023-08-15
Supported by
National Natural Science Foundation of China(21662020); National Natural Science Foundation of China(21901091); Academician (Expert) Workstation Project, Wang Yuan Chao Expert Workstation in Yunnan Province(202305AF150018)
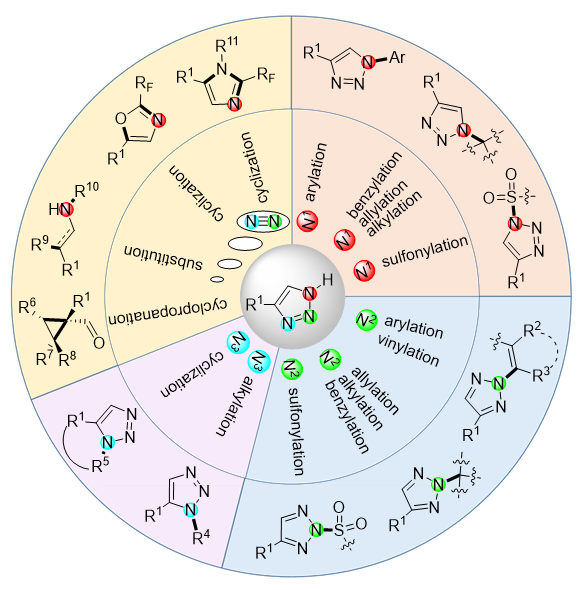
1,2,3-三氮唑是一类广泛应用在医药、农药以及材料等领域的五元氮杂环结构. 自2002年Sharpless和Meldal等发展了铜催化叠氮-炔偶极环加成反应以来, 1,2,3-三唑化学在有机合成和药物化学领域的应用进入了新的发展阶段. 4-单取代-1,2,3-三氮唑是该类化合物家族中的重要成员之一, 其结构具有易于修饰转化的优点, 由此可获得结构新颖、种类多样的衍生物分子. 主要综述了4-单取代-1,2,3-三氮唑在结构修饰方面的研究进展, 转化情况根据反应位点的不同分别展开, 包括N1、N2以及N3位, 并讨论了反应的底物范围、局限性以及代表性反应机理.
杨保民 , 张水滔 , 董鲜 , 秦贵平 , 江玉波 . 4-单取代-1,2,3-三氮唑的修饰转化研究进展[J]. 化学学报, 2023 , 81(11) : 1577 -1589 . DOI: 10.6023/A23060284
1,2,3-Triazole is a kind of 5-membered N-heterocycle widely applied in pharmaceutical, pesticide and materials. 1,2,3-Trizole chemistry has stepped into a new era in the fields of organic synthesis and pharmacochemistry since the Cu-catalyzed azide-alkyne dipolar cycloaddition was founded by the Sharpless and Meldal groups in 2002. 4-Monosubstituted 1,2,3-triazole is a noble member in its family owing to the structural merits of facile modifications, leading to novel molecules. The transformations of 4-monosubstituted 1,2,3-triazoles basing on different reaction sites including the N1, N2 and N3 are reviewed. The substrate scope, limitation, and representative mechanism are also discussed.

| [1] | (a) Vitaku E.; Smith D. T.; Njardarson J. T. J. Med. Chem. 2014, 57, 10257. |
| [1] | (b) Liu X.; Kuang C.; Su C. Acta Chim. Sinica 2022, 80, 1135. (in Chinese) |
| [1] | ( 刘霞, 匡春香, 苏长会, 化学学报, 2022, 80, 1135.) |
| [2] | (a) Suzuki T.; Ota Y.; Ri M.; Bando M.; Gotoh A.; Itoh Y.; Tsumoto H.; Tatum P. R.; Mizukami T.; Nakagawa H. J. Med. Chem. 2012, 55, 9562. |
| [2] | (b) Sun L.; Huang T.; Dick A.; Meuser M. E.; Zalloum W. A.; Chen C.-H.; Ding X.; Gao P.; Cocklin S.; Lee K.-H.; Zhan P.; Liu X. Eur. J. Med. Chem. 2020, 190, 112085. |
| [2] | (c) Othman E. M.; Fayed E. A.; Husseiny E. M.; Abulkhair H. S. Bioorg. Chem. 2022, 123, 105762. |
| [2] | (d) Xiao L.; Shi D. Chin. J. Org. Chem. 2010, 30, 85. (in Chinese) |
| [2] | ( 肖琳霞, 石德清, 有机化学, 2010, 30, 85.) |
| [2] | (e) Chen Z.; Jiang Y.; Xu C.; Sun X.; Ma C.; Xia Z.; Zhao H. Molecules 2022, 27, 4928. |
| [2] | (f) Dong Y.; Hu X.; Duan C.; Liu P.; Liu S.; Lan L.; Chen D.; Ying L.; Su S.; Gong X.; Huang F.; Cao Y. Adv. Mater. 2013, 25, 3683. |
| [2] | (g) Zhou J.; Lei P.; Geng Y.; He Z.; Li X.; Zeng Q.; Tang A.; Zhou E. J. Mater. Chem. A 2022, 10, 9869. |
| [3] | (a) Huisgen R. Angew. Chem., Int. Ed. 1963, 2, 565. |
| [3] | (b) Huisgen R. Angew. Chem., Int. Ed. 1963, 2, 633. |
| [3] | (c) Huisgen R. Pure Appl. Chem. 1989, 61, 613. |
| [3] | (d) Qiu K.; Li J.; Ma H.; Zhou W.; Cai Q. Acta Chim. Sinica 2023, 81, 42. (in Chinese) |
| [3] | ( 邱孔茜, 李杰, 马浩文, 周伟, 蔡倩, 化学学报, 2023, 81, 42.) |
| [4] | (a) Rostovtsev V. V.; Green L. G.; Fokin V. V.; Sharpless K. B. Angew. Chem., Int. Ed. 2002, 41, 2596. |
| [4] | (b) Torn?e C. W.; Christensen C.; Meldal M. J. Org. Chem. 2002, 67, 3057. |
| [4] | (c) Meldal M.; Torn?e C. W. Chem. Rev. 2008, 108, 2952. |
| [4] | (d) Xu L.; Dong J. Chin. J. Chem. 2020, 38, 414. |
| [4] | (e) Schulze B.; Schubert U. S. Chem. Soc. Rev. 2014, 43, 2522. |
| [5] | (a) Liu Z.; Ji H.; Gao W.; Zhu G.; Tong L.; Lei F.; Tang B. Chem. Commun. 2017, 53, 6259. |
| [5] | (b) Ji J.; Guan C.; Wei Q.; Chen X.; Zhao Y.; Liu S. Org. Lett. 2022, 24, 132. |
| [5] | (c) Zhu L.; Zhang H.; Wang C.; Chen Z. Chin. J. Org. Chem. 2018, 38, 1052. (in Chinese) |
| [5] | ( 朱莉莉, 张辉, 王春杰, 陈自立, 有机化学, 2018, 38, 1052.) |
| [6] | (a) Zhang W.; Kuang C.; Yang Q. Chin. J. Org. Chem. 2011, 31, 54. (in Chinese) |
| [6] | ( 张文生, 匡春香, 杨青, 有机化学, 2011, 31, 54.) |
| [6] | (b) Agalave S. G.; Maujan S. R.; Pore V. S. Chem.-Asian J. 2011, 6, 2696. |
| [7] | McAllister L. A.; Montgomery J. I.; Abramite J. A.; Reilly U.; Brown M. F.; Chen J. M.; Barham R. A.; Che Y.; Chung S. W.; Menard C. A.; Mark M.-F.; Mullins L. M.; Noe M. C.; Donnell J. P.; Oliver R. M. III; Penzien J. B.; Plummer M.; Price L. M.; Shanmugasundaram V.; Tomaras A. P.; Uccello D. P. Bioorg. Med. Chem. Lett. 2012, 22, 6832. |
| [8] | Hsu K.-L.; Tsuboi K.; Whitby L. R.; Speers A. E.; Pugh H.; Inloes J.; Cravatt B. F. J. Med. Chem. 2013, 56, 8257. |
| [9] | Thirumurugan P.; Matosiuk D.; Jozwiak K. Chem. Rev. 2013, 113, 4905. |
| [10] | Saroha B.; Kumar G.; Kumar R.; Kumari M.; Kumar S. Chem. Biol. Drug Des. 2021, 100, 843. |
| [11] | Umapathi N.; Jalapathi P.; Raghavender M.; Shankar B. Asian J. Chem. 2021, 33, 2341. |
| [12] | Natarajan R.; Kesavan Y.; Sivaperuman A.; Subramani A. Curr. Bioact. Compd. 2022, 18, e070322201800. |
| [13] | (a) Chen Y.; Liu Y.; Petersen J. L.; Shi X. Chem. Commun. 2008, 28, 3254. |
| [13] | (b) Liu Y.; Yan W.; Chen Y.; Petersen J. L.; Shi X. Org. Lett. 2008, 10, 5389. |
| [13] | (c) Madadi N. R.; Penthala N. R.; Song L.; Hendrickson H. P.; Crooks P. A. Tetrahedron Lett. 2014, 55, 4207. |
| [13] | (d) Madadi N. R.; Penthala N. R.; Howk K.; Ketkar A.; Eoff R. L.; Borrelli M. J.; Crooks P. A. Eur. J. Med. Chem. 2015, 103, 123. |
| [13] | (e) Reddy R. J.; Waheed M.; Karthik T.; Shankar A. New J. Chem. 2018, 42, 980. |
| [13] | (f) Yang J.; Yin W.; Liu R.; Chu C. Chin. J. Chem. 2012, 30, 2786. |
| [14] | Wang J.; Yu J.; Chen J.; Jiang Y.; Xiao T. Org. Biomol. Chem. 2021, 19, 6974. |
| [15] | Zhao S.; Liu J.; Lv Z.; Zhang G.; Xu Z. Eur. J. Med. Chem. 2023, 251, 115254. |
| [16] | Thottempudi V.; Yin P.; Zhang J.; Parrish D. A.; Shreeve J. M. Chem.-Eur. J. 2014, 20, 542. |
| [17] | Franco C. A.; da Silva T. I.; Dias M. G.; Ferreira B. W.; de Sousa B. L.; Bousada G. M.; Barreto R. W.; Vaz B. G.; Lima G. S.; Santos M. H. D.; Grossi J. A. S.; Varej?o E. V. V. J. Agric. Food Chem. 2022, 70, 2806. |
| [18] | (a) Liao Y.; Lu Q.; Chen G.; Yu Y.; Li C.; Huang X. ACS Catal. 2017, 7, 7529. |
| [18] | (b) Kumar N.; Bhadoria D.; Kumar A. Green Chem. 2021, 23, 7987. |
| [18] | (c) Fang D.; Zhang Z.-Y.; Shangguan Z.; He Y.; Yu C.; Li T. J. Am. Chem. Soc. 2021, 143, 14502. |
| [18] | (d) Zeng L.; Lai Z.; Zhang C.; Xie H.; Cui S. Org. Lett. 2020, 22, 2220. |
| [18] | (e) Duan X.; Zheng N.; Liu G.; Li M.; Wu Q.; Sun X.; Song W. Org. Lett. 2022, 24, 6006. |
| [18] | (f) Guo W.-T.; Zhu B.-H.; Chen Y.; Yang J.; Qian P.-C.; Deng C.; Ye L.-W.; Li L. J. Am. Chem. Soc. 2022, 144, 6981. |
| [18] | (g) Zhang X.; Li S.; Yu W.; Xie Y.; Tung C.-H.; Xu Z. J. Am. Chem. Soc. 2022, 144, 6200. |
| [18] | (h) Qin C.-Q.; Zhao C.; Chen G.-S.; Liu Y.-L. ACS Catal. 2023, 13, 6301. |
| [18] | (i) Rahul P.; Thomas J.; Dehaen W.; John J. Molecules 2023, 28, 308. |
| [18] | (j) Wei F.; Wang W.; Ma Y.; Tung C.-H.; Xu Z. Chem. Commun. 2016, 52, 14188. |
| [18] | (k) Spiteri C.; Moses J. E. Angew. Chem., Int. Ed. 2010, 49, 31. |
| [19] | Samanta S.; Ravi C.; Rao S. N.; Joshi A.; Adimurthy S. Org. Biomol. Chem. 2017, 15, 9590. |
| [20] | Yi H.; Chen H.; Bian C.; Tang Z.; Singh A. K.; Qi X.; Yue X.; Lan Y.; Lee J.-F.; Lei A. Chem. Commun. 2017, 53, 6736. |
| [21] | Li T.; Chen B.-L.; Zhu L.-L.; Chen Z. Tetrahedron Lett. 2020, 61, 151851. |
| [22] | Chao Z.; Ma M.; Gu Z. Org. Lett. 2020, 22, 6441. |
| [23] | Berthold D.; Breit B. Org. Lett. 2018, 20, 598. |
| [24] | Yang Y.-Z.; Song R.-J.; Li J.-H. Org. Lett. 2019, 21, 3228. |
| [25] | Tiwari V.; Bingham J. T.; Vyas S.; Singh A. Org. Biomol. Chem. 2020, 18, 9044. |
| [26] | Rai V.; Kavyashree P.; Harmalkar S. S.; Dhuri S. N.; Maddani M. R. Org. Biomol. Chem. 2022, 20, 345. |
| [27] | Zhang L.; Yi H.; Wang J.; Lei A. J. Org. Chem. 2017, 82, 10704. |
| [28] | Wu J.; Zhou Y.; Zhou Y.; Chiang C.-W.; Lei A. ACS Catal. 2017, 7, 8320. |
| [29] | Wu J.; Zhu J.; Yu L.; Yu X.; Li W.; Xie L.; Wu J.; Li Z. Asian J. Org. Chem. 2022, 11, e202200551. |
| [30] | Tan Z.; Xiang F.; Xu K.; Zeng C. Org. Lett. 2022, 24, 5345. |
| [31] | Tang Z.-L.; Ouyang X.-H.; Song R.-J.; Li J.-H. Org. Lett. 2021, 23, 1000. |
| [32] | Wang C.; Ji X.; Deng G.-J.; Huang H. Org. Biomol. Chem. 2022, 20, 1200. |
| [33] | Beryozkina T. V.; Efimov I. V.; Fabian W. M. F.; Beliaev N. A.; Slepukhin P. A.; Isenov M. L.; Dehaen W.; Lubec G.; Eltsov O. S.; Fan Z.; Thomas J.; Bakulev V. A. Tetrahedron 2015, 71, 6189. |
| [34] | Ueda S.; Su M.; Buchwald S. L. Angew. Chem., Int. Ed. 2011, 50, 8944. |
| [35] | Lopes A. B.; Wagner P.; Kümmerle A. E.; Bihel F.; Bourguignon J.-J.; Schmitt M.; Miranda L. S. M. ChemistrySelect 2017, 2, 6544. |
| [36] | Wen J.; Zhu L.-L.; Bi Q.-W.; Shen Z.-Q.; Li X.-X.; Li X.; Wang Z.; Chen Z. Chem.-Eur. J. 2014, 20, 974. |
| [37] | Gu C.-X.; Bi Q.-W.; Gao C.-K.; Wen J.; Zhao Z.-G.; Chen Z. Org. Biomol. Chem. 2017, 15, 3396. |
| [38] | Roshandel S.; Lunn M. J.; Rasul G.; Ravinson D. S. M.; Suri S. C.; Prakash G. K. S. Org. Lett. 2019, 21, 6255. |
| [39] | Yan W.; Liao T.; Tuguldur O.; Zhong C.; Petersen J. L.; Shi X. Chem.-Asian J. 2011, 6, 2720. |
| [40] | Bhagat U. K.; Kamaluddin; Peddinti R. K.; Tetrahedron Lett. 2017, 58, 298. |
| [41] | Bhagat U. K.; Peddinti R. K. J. Org. Chem. 2018, 83, 793. |
| [42] | Tang S.; Yu J.; Shao Y.; Sun J. Org. Chem. Front. 2021, 8, 278. |
| [43] | Bhagat U. K.; Kamaluddin; Peddinti R. K. Synthesis 2017, 49, 3985. |
| [44] | Luo G.; Sun C.; Li Y.; Li X.; Zhao Z. RSC Adv. 2018, 8, 27610. |
| [45] | Deng X.; Lei X.; Nie G.; Jia L.; Li Y.; Chen Y. J. Org. Chem. 2017, 82, 6163. |
| [46] | Aruri H.; Singh U.; Kumar M.; Sharma S.; Aithagani S. K.; Gupta V. K.; Mignani S.; Vishwakarma R. A.; Singh P. P. J. Org. Chem. 2017, 82, 1000. |
| [47] | Gupta S.; Chandna N.; Singh A. K.; Jain N. J. Org. Chem. 2018, 83, 3226. |
| [48] | Zhang C.; Zheng L.; Yan Q.; Hu Q.; Jia F.; Chen Y. ChemistrySelect 2018, 3, 10277. |
| [49] | Rajamanickam S.; Saraswat M.; Venkataramani S.; Patel B. K. Chem. Sci. 2021, 12, 15318. |
| [50] | Zhu L.-L.; Xu X.-Q.; Shi J.-W.; Chen B.-L.; Chen Z. J. Org. Chem. 2016, 81, 3568. |
| [51] | Wei H.; Hu Q.; Ma Y.; Wei L.; Liu J.; Shi M.; Wang F. Asian J. Org. Chem. 2017, 6, 662. |
| [52] | Bhagat U. K.; Peddinti R. K. Synlett 2018, 29, 99. |
| [53] | Zhu L.-L.; Tian L.; Cai B.; Liu G.; Zhang H.; Wang Y. Chem. Commun. 2020, 56, 2979. |
| [54] | Zhu L.-L.; Tian L.; Sun K.; Li Y.; Liu G.; Cai B.; Zhang H.; Wang Y. J. Org. Chem. 2022, 87, 12963. |
| [55] | Stivanin M. L.; Fernandes A. A. G.; da Silva A. F.; Okada Jr C. Y.; Jurberg I. D. Adv. Synth. Catal. 2020, 362, 1106. |
| [56] | Man X. J. A. T. R. H.; Liu Y. C.; Li X. X.; Zhao Z. G. New J. Chem. 2019, 43, 14739. |
| [57] | Duan H.; Yan W.; Sengupta S.; Shi X. Bioorg. Med. Chem. Lett. 2009, 19, 3899. |
| [58] | Motornov V.; Latyshev G. V.; Kotovshchikov Y. N.; Lukashev N. V.; Beletskaya I. P. Adv. Synth. Catal. 2019, 361, 3306. |
| [59] | Yan W.; Wang Q.; Chen Y.; Petersen J. L.; Shi X. Org. Lett. 2010, 12, 3308. |
| [60] | Yan W.; Ye X.; Weise K.; Petersen J. L.; Shi X. Chem. Commun. 2012, 48, 3521. |
| [61] | Reddy R. J.; Shankar A.; Waheed M.; Nanubolu J. B. Tetrahedron Lett. 2018, 59, 2014. |
| [62] | Desai S. P.; Zambri M. T.; Taylor M. S. J. Org. Chem. 2022, 87, 5385. |
| [63] | Chen Y.; Zhou S.; Ma S.; Liu W.; Pan Z.; Shi X. Org. Biomol. Chem. 2013, 11, 8171. |
| [64] | Duan S.; Chen Y.; Meng H.; Shan L.; Xu Z.-F.; Li C.-Y. Asian J. Org. Chem. 2021, 10, 224. |
| [65] | Zhang S.; Li J.; Xiao T.; Yang B.; Jiang Y. Molecules 2022, 27, 7567. |
| [66] | Grimster N.; Zhang L.; Fokin V. V. J. Am. Chem. Soc. 2010, 132, 2510. |
| [67] | Wang T.; Tang Z.; Luo H.; Tian Y.; Xu M.; Lu Q.; Li B. Org. Lett. 2021, 23, 6293. |
| [68] | Motornov V.; Beier P. Org. Lett. 2022, 24, 1958. |
| [69] | Motornov V.; Beier P. New J. Chem. 2022, 46, 14318. |
/
| 〈 |
|
〉 |