

基于尼罗红类ONOO–近红外荧光探针的开发及其成像应用
收稿日期: 2023-05-13
网络出版日期: 2023-08-15
基金资助
国家自然科学基金(22277098); 国家自然科学基金(21904105); 陕西化生基础科学研究项目(22JHQ070)
Development of a Near-Infrared Fluorescent Probe Based on Nile Red for ONOO– and Its Imaging Applications
Received date: 2023-05-13
Online published: 2023-08-15
Supported by
National Natural Science Foundation of China(22277098); National Natural Science Foundation of China(21904105); Shaanxi Fundamental Science Research Project for Chemistry & Biology(22JHQ070)
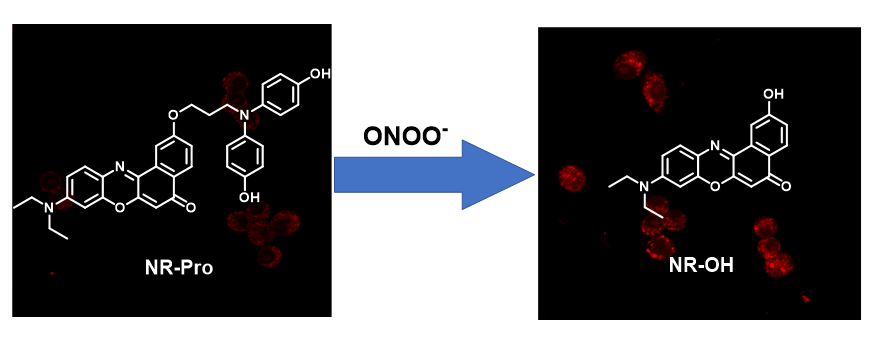
过氧亚硝酸根(ONOO–)在氧化应激和炎症过程中的生成与细胞损伤、炎症反应和免疫调节等密切相关, 因此, 开发高灵敏且高度选择性的ONOO–检测方法对于深入理解疾病发生和发展机制、提高疾病的早期诊断和治疗水平具有极其重要意义. 设计合成了一种选择性检测ONOO–的新型荧光探针NR-Pro. 该探针通过使用烷基链将2-羟基尼罗红衍生物(NR-OH)与4,4'-氮杂二基二苯酚基团有机结合, 在ONOO–作用下, 释放出近红外染料NR-OH. 实验研究表明, 该探针在658 nm处对ONOO–表现出显著的“OFF-ON”型荧光信号响应, 且对ONOO–浓度在0~10 μmol/L范围内呈现良好的线性关系, 检出限低至17.7 nmol/L. 这项研究进一步证实了NR-Pro探针在检测RAW264.7细胞中外源和内源ONOO–方面具有优良的成像能力, 这为早期诊断和治疗疾病提供了新的思路.
贺晓梦 , 袁方 , 张素雅 , 张健健 . 基于尼罗红类ONOO–近红外荧光探针的开发及其成像应用[J]. 化学学报, 2023 , 81(11) : 1515 -1521 . DOI: 10.6023/A23050225
The production of peroxynitrite (ONOO–) in situations of oxidative stress and inflammation has a profound impact on cellular damage, inflammatory responses, and immune regulation. Thus, the advancement of highly sensitive and selective techniques to detect ONOO– is of great significance. These methods are crucial for gaining insights into disease mechanisms, improving early disease diagnosis, and enhancing treatment effectiveness. In this study, we have designed and synthesized a novel fluorescent probe, NR-Pro, specifically for the selective detection of ONOO–. The probe incorporates a 2-hydroxy Nile red derivative (NR-OH) that is organically bound to a 4,4'-azabediyldiphenol group via an alkyl chain. Upon interaction with ONOO–, the probe releases the near-infrared dye NR-OH, leading to a significant “OFF-ON” fluorescence signal response at 658 nm. The mechanism involves the initial attack of ONOO– on the 4,4'-aza-diyl diphenol group, resulting in the formation of an alkylamine-modified Nile red derivative and the release of two 1,4-benzoquinone molecules. Subsequently, the alkylamine group undergoes further oxidation by ONOO–, leading to the liberation of the near-infrared fluorophore NR-OH and acrolein through a 1,3-elimination reaction with the involvement of water. Experimental investigations have demonstrated that the probe exhibits a favorable linear relationship with ONOO– concentration in the range of 0 to 10 μmol/L, with an exceptionally low detection limit of 17.7 nmol/L. Furthermore, the fluorescence emission of NR-Pro remained unaffected by various biological species, including reactive oxygen species (ROSs), metal ions, enzymes, anions, and amino acids. NR-Pro also exhibited minimal cytotoxicity and very high photostability, suggesting its potential suitability for cell imaging applications. Moreover, our study confirms the remarkable imaging capability of the NR-Pro probe in detecting both exogenous and endogenous ONOO– in RAW264.7 cells. These findings present novel insights for the early diagnosis and treatment of diseases, paving the way for potential advancements in the field.

Key words: near-infrared; fluorescent probe; peroxynitrite; Nile red; cell imaging
| [1] | Nathan C.; Cunningham-Bussel A. Nat. Rev. Immunol. 2013, 13, 349. |
| [2] | Dickinson B. C.; Chang C. J. Nat. Chem. Biol. 2011, 7, 504. |
| [3] | Liu Y.; Teng L.; Lyu Y.; Song G.; Zhang X. B.; Tan W. Nat. Commun. 2022, 13, 2216. |
| [4] | Wang Y.; Shi L.; Ye Z.; Guan K.; Teng L.; Wu J.; Yin X.; Song G.; Zhang X. B. Nano. Lett. 2020, 20, 176. |
| [5] | Ferrer-Sueta G.; Campolo N.; Trujillo M.; Bartesaghi S.; Carballal S.; Romero N.; Alvarez B.; Radi R. Chem. Rev. 2018, 118, 1338. |
| [6] | Radi R. J. Biol. Chem. 2013, 288, 26464. |
| [7] | De Armas M. I.; Esteves R.; Viera N.; Reyes A. M.; Mastrogiovanni M.; Alegria T. G. P.; Netto L. E. S.; Tortora V.; Radi R.; Trujillo M. Free Radic. Biol. Med. 2019, 130, 369. |
| [8] | Graham P. M.; Li J. Z.; Dou X.; Zhu H.; Misra H. P.; Jia Z.; Li Y. Mol. Cell Biochem. 2013, 378, 291. |
| [9] | Li X.; Tao R. R.; Hong L. J.; Cheng J.; Jiang Q.; Lu Y. M.; Liao M. H.; Ye W. F.; Lu N. N.; Han F.; Hu Y. Z.; Hu Y. H. J. Am. Chem. Soc. 2015, 137, 12296. |
| [10] | Islam M. T. Neurol. Res. 2017, 39, 73. |
| [11] | Munn L. L. WIREs Syst. Biol. Med. 2017, 9, e1382. |
| [12] | Xie X.; Tang F.; Liu G.; Li Y.; Su X.; Jiao X.; Wang X.; Tang B. Anal. Chem. 2018, 90, 11629. |
| [13] | Bartesaghi S.; Radi R. Redox Biol. 2018, 14, 618. |
| [14] | Hu J. S.; Shao C.; Wang X.; Di X.; Xue X.; Su Z.; Zhao J.; Zhu H. L.; Liu H. K.; Qian Y. Adv. Sci. 2019, 6, 1900341. |
| [15] | Gao L.; Wang W.; Wang X.; Yang F.; Xie L.; Shen J.; Brimble M. A.; Xiao Q.; Yao S. Q. Chem. Soc. Rev. 2021, 50, 1219. |
| [16] | Zhang S. Y.; Ning L. L.; Song Z. H.; Zhao X. Y.; Guan F.; Yang X. F.; Zhang J. J. Anal. Chem. 2022, 94, 5805. |
| [17] | Zhao X. Y.; Ning L. L.; Zhou X. M.; Song Z. H.; Zhang J. J.; Guan F.; Yang X. F. Anal. Chem. 2021, 93, 4894. |
| [18] | Wu W.; Zhang C.; Rees T. W.; Liao X.; Yan X.; Chen Y.; Ji L.; Chao H. Anal. Chem. 2020, 92, 6003. |
| [19] | Ueno T.; Nagano T. Nat. Methods 2011, 8, 642. |
| [20] | Chan J.; Dodani S. C.; Chang C. J. Nat. Chem. 2012, 4, 973. |
| [21] | Zhang S.-Y.; Zhang J.-J. Fine Chem. 2020, 37, 2229. (in Chinese) |
| [21] | ( 张素雅, 张健健, 精细化工, 2020, 37, 2229.) |
| [22] | Li Y.; Ning L. L.; Yuan F.; Zhang T.; Zhang J. J.; Xu Z. G.; Yang X. F. Anal. Chem. 2020, 92, 5733. |
| [23] | Zhao X. Y.; Ding M. B.; Ning L. L.; Yuan F.; Li J. C.; Guo Y.; Mu Y. G.; Zhang J. J. Acta Mater. Med. 2022, 1, 476. |
| [24] | Yuan F.; He X. M.; Lu Y. R.; Ning L. L.; Zhao X. Y.; Zhang S. Y.; Guan F.; Guo Y.; Zhang J. J. Anal. Chem. 2023, 95, 6931. |
| [25] | Lv X.; Wu Y.; Zhang B.-R.; Guo W. Acta Chim. Sinica 2023, 81, 359. (in Chinese) |
| [25] | ( 吕鑫, 吴仪, 张勃然, 郭炜, 化学学报, 2023, 81, 359.) |
| [26] | Li X.; Liang X.; Yin J.; Lin W. Chem. Soc. Rev. 2021, 50, 102. |
| [27] | Lu J.; Li Z.; Gao Q.; Tan J.; Sun Z.; Chen L.; You J. Anal. Chem. 2021, 93, 3426. |
| [28] | Wang N.; Wang H.; Zhang J.; Ji X.; Su H.; Liu J.; Wang J.; Zhao W. Chin. Chem. Lett. 2022, 33, 1584. |
| [29] | Cui J.; Zang S.; Nie H.; Shen T.; Su S.; Jing J.; Zhang X. Sensor. Actuat. B-Chem. 2021, 328, 129069. |
| [30] | Chen S.; Vurusaner B.; Pena S.; Thu C. T.; Mahal L. K.; Fisher E. A.; Canary J. W. Anal. Chem. 2021, 93, 10090. |
| [31] | Huang J.; Wang C.; Lin M.-G.; Zeng F.; Wu S.-Z. Acta Chim. Sinica 2021, 79, 331. (in Chinese) |
| [31] | ( 黄靖, 王超, 林敏刚, 曾钫, 吴水珠, 化学学报, 2021, 79, 331.) |
| [32] | Chen F.; Teng L.; Lu C.; Zhang C.; Rong Q.; Zhao Y.; Yang Y.; Wang Y.; Song G.; Zhang X. Anal. Chem. 2020, 92, 13452. |
| [33] | Zhou D. Y.; Li Y.; Jiang W. L.; Tian Y.; Fei J.; Li C. Y. Chem. Commun. 2018, 54, 11590. |
| [34] | Cheng D.; Peng J.; Lv Y.; Su D.; Liu D.; Chen M.; Yuan L.; Zhang X. J. Am. Chem. Soc. 2019, 141, 6352. |
| [35] | Martinez V.; Henary M. Chemistry 2016, 22, 13764. |
| [36] | Jose J.; Burgess K. Tetrahedron 2006, 62, 11021. |
| [37] | Sebok-Nagy K.; Miskolczy Z.; Biczok L. Photochem. Photobiol. 2005, 81, 1212. |
| [38] | Wang P.; Yu L.; Gong J.; Xiong J.; Zi S.; Xie H.; Zhang F.; Mao Z.; Liu Z.; Kim J. S. Angew. Chem., Int. Ed. 2022, 61, e202206894. |
| [39] | Lin K. K.; Wu S. C.; Hsu K. M.; Hung C. H.; Liaw W. F.; Wang Y. M. Org. Lett. 2013, 15, 4242. |
| [40] | Hooper D. C.; Scott G. S.; Zborek A.; Mikheeva T.; Kean R. B.; Koprowski H.; Spitsin S. V. FASEB J. 2000, 14, 691. |
| [41] | Wang Z.; Cong T. D.; Zhong W.; Lau J. W.; Kwek G.; Chan-Park M. B.; Xing B. Angew. Chem., Int. Ed. 2021, 60, 16900. |
/
| 〈 |
|
〉 |