

混合工艺对氧化物/硫化物复合固态电解质电化学性能的影响
Effect of Mixing Strategy on Electrochemical Performance of Oxide/Sulfide Solid Electrolyte
Received date: 2023-05-05
Online published: 2023-08-15
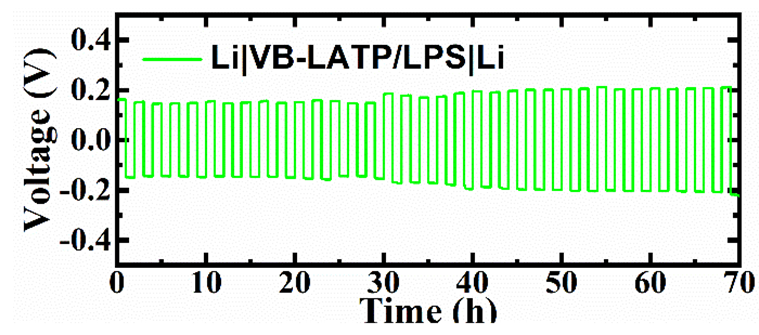
随着新能源汽车的普及, 全固态电池由于其高安全性和高能量密度而受到广泛关注. 氧化物/硫化物复合固态电解质兼具硫化物的低晶界电阻、室温加工性与界面电阻以及氧化物优异的电化学稳定性和低成本等优点, 成为研究的热点. 但是目前复合固态电解质中氧化物的质量分数过低(w<10%), 限制了成本的进一步下降和稳定性的进一步提高. 以Li1.3Al0.3Ti1.7(PO4)3 (LATP)/Li8P2S9 (LPS)复合电解质作为示例性体系, 通过对不同混合工艺的探究, 成功制备了氧化物粒径细小且分散均匀的复合固态电解质, 获得更高的离子电导率和对锂循环性能. 以此组装的全固态电池在100次循环后, 容量保持率高达99%, 显示出卓越的电化学稳定性.
张冠华 , 杨子涵 , 马越 . 混合工艺对氧化物/硫化物复合固态电解质电化学性能的影响[J]. 化学学报, 2023 , 81(10) : 1387 -1393 . DOI: 10.6023/A23050203
With the widespread development of new energy vehicles, all-solid-state batteries have attracted wide attention because of their high safety and high energy density. The oxide/sulfide solid electrolyte is expected to combine the low grain boundary resistance, room temperature workability and low interfacial resistance of sulfide with the excellent electrochemical stability and low cost of oxides. However, the lack of reliable preparation techniques for composite solid electrolytes with higher oxide content limits the further reduction of cost and the further improvement of stability. In this work, Li1.3Al0.3Ti1.7(PO4)3 (LATP)/Li8P2S9 (LPS) electrolyte was employed as an example system for the synthesis of sulfide-based solid electrolyte with high oxide content via grinding and subsequent hot compressing. The LATP and LPS was mixed through normal grinding (Gr), low speed ball grinding (LB) and variable speed ball grinding (VB). The results showed that grain refinement of oxide and the decrease of pore content were achieved by VB. In addition, the distribution of S and Ti elements proved that LATP was uniformly dispersed in the VB-LATP/LPS (LATP/LPS prepared by VB). According to the X-ray diffraction (XRD) pattern, the distortion of LATP and LPS lattice in VB-LATP/LPS was attributed to the mutual diffusion of oxygen and sulfur atoms at the interface. As a result, VB-LATP/LPS exhibited high lithium ion conductivity (3.35 mS•cm-1), low electron conductivity (1.53×10-8 S•cm-1) and relatively low lithium ion migration activation energy (11.75 kJ•mol-1) at room temperature. Besides, the good interfacial bonding state and addition of hard oxides contributed to the high stability of the electrolyte/lithium interface. Furthermore, the all-solid-state battery assembled by VB-LATP/LPS showed a high capacity retention rate of 99% after 100 cycles, demonstrating excellent electrochemical stability. Such synthesis idea of combination with soft sulfide electrolyte and hard oxide electrolyte provides a feasible strategy for the synthesis of cost effective composite solid electrolytes.

| [1] | Tian, S. W.; Zhou, L. X.; Zhang, B. Q.; Zhang, J. J.; Du, X. F.; Zhang, H.; Hu, S. J.; Yuan, Z. X.; Han, P. X.; Li, S. L.; Zhao, W.; Zhou, X. H.; Cui, G. L. Acta Chim. Sinica 2022, 80, 1410 (in Chinese). |
| [1] | (田宋炜, 周丽雪, 张秉乾, 张建军, 杜晓璠, 张浩, 胡思伽, 苑志祥, 韩鹏献, 李素丽, 赵伟, 周新红, 崔光磊, 化学学报, 2022, 80, 1410.) |
| [2] | Zheng, L.; Yi, R.; Zheng, N.; Shen, Y.; Chen, L. Chin. J. Chem. 2023, 41, 814. |
| [3] | Qu, X.; Guo, Y.; Liu, X. Chin. J. Chem. 2022, 40, 2559. |
| [4] | Zhang, X.; Liu, R.; Wang, L.; Fu, H. Acta Chim. Sinica 2021, 79, 670 (in Chinese). |
| [4] | (张欣欣, 刘荣, 王蕾, 付宏刚, 化学学报, 2021, 79, 670.) |
| [5] | Li, Z.; Peng, Z.; Sun, R.; Qin, Z.; Liu, X.; Wang, C.; Fan, H.; Lu, S. Chin. J. Chem. 2021, 39, 2599. |
| [6] | Kamaya, N.; Homma, K.; Yamakawa, Y.; Hirayama, M.; Kanno, R.; Yonemura, M.; Kamiyama, T.; Kato, Y.; Hama, S.; Kawamoto, K.; Mitsui, A. Nat. Mater. 2011, 10, 682. |
| [7] | Liang, Q.; Guo, Y.; Guo, J.; Xiang, M.; Liu, X.; Bai, W.; Ning, P. Acta Chim. Sinica 2021, 79, 1526 (in Chinese). |
| [7] | (梁其梅, 郭昱娇, 郭俊明, 向明武, 刘晓芳, 白玮, 宁平, 化学学报, 2021, 79, 1526.) |
| [8] | Li, Y.; Wu, Y.; Ma, T.; Wang, Z.; Gao, Q.; Xu, J.; Chen, L.; Li, H.; Wu, F. Adv. Energy Mater. 2022, 12, 2201732. |
| [9] | Ma, T. H.; Wang, Z. X.; Wu, D. X.; Lu, P. S.; Zhu, X.; Yang, M.; Peng, J.; Chen, L. Q.; Li, H.; Wu, F. Energy Environ. Sci. 2023, 16, 2142. |
| [10] | Sun, C. W.; Liu, J.; Gong, Y. D.; Wilkinson, D. P.; Zhang, J. J. Nano Energy 2017, 33, 363. |
| [11] | Zhang, Q.; Cao, D. X.; Ma, Y.; Natan, A.; Aurora, P.; Zhu, H. L. Adv. Mater. 2019, 31. |
| [12] | Jia, Z.; Zhang, X.; Qian, M.; Jin, Y.; Xiong, Y. Electrochem. Commun. 2023, 147, 107438. |
| [13] | Yang, X.; Luo, J.; Sun, X. Chem. Soc. Rev. 2020, 49, 2140. |
| [14] | Seino, Y.; Ota, T.; Takada, K.; Hayashi, A.; Tatsumisago, M. Energy Environ. Sci. 2014, 7, 627. |
| [15] | Jiang, P. F.; Du, G. Y.; Cao, J. Q.; Zhang, X. Y.; Zou, C. C.; Liu, Y. T.; Lu, X. Energy Technol-Ger. 2023, 11, 2201288. |
| [16] | Kwak, H.; Wang, S.; Park, J.; Liu, Y. S.; Kim, K. T.; Choi, Y.; Mo, Y. F.; Jung, Y. S. ACS Energy Lett. 2022, 7, 1776. |
| [17] | Johari, S.; Tajuddin, N. A.; Hanibah, H.; Deraman, S. K. Int. J. Electrochem. Sci. 2021, 16, 211049. |
| [18] | Tian, J. X.; Guo, H. J.; Wan, J.; Liu, G. X.; Yan, H. J.; Wen, R.; Wan, L. J. Acta Chim. Sinica 2021, 79, 1197 (in Chinese). |
| [18] | (田建鑫, 郭慧娟, 万静, 刘桂贤, 严会娟, 文锐, 万立骏, 化学学报, 2021, 79, 1197.) |
| [19] | Liang, S.; Kang, S.; Yang, D.; Hu, J. Acta Chim. Sinica 2022, 80, 1264 (in Chinese). |
| [19] | (梁世硕, 康树森, 杨东, 胡建华, 化学学报, 2022, 80, 1264.) |
| [20] | Li, M. R.; Kolek, M.; Frerichs, J. E.; Sun, W.; Hou, X.; Hansen, M. R.; Winter, M.; Bieker, P. ACS Sustainable Chem. Eng. 2021, 9, 11314. |
| [21] | Cao, C. C.; Zhong, Y. J.; Shao, Z. P. Chin. J. Chem. 2023, 41, 1119. |
| [22] | Rangasamy, E.; Sahu, G.; Keum, J. K.; Rondinone, A. J.; Dudney, N. J.; Liang, C. D. J. Mater. Chem. A 2014, 2, 4111. |
| [23] | Jia, Z.; Zhang, X.; Qian, M.; Jin, Y.; Xiong, Y. Chem. Eng. J. 2022, 435, 134663. |
| [24] | Baltash, Y.; Mashekova, A.; Yegamkulov, M.; Trussov, I.; Bakenov, Z.; Mukanova, A. Ionics 2023, 29, 2647. |
| [25] | Liu, D.; Wang, Q.; Ma, X.; Liu, Q.; Zhou, X.; Lei, Z. J. Alloy Compd. 2022, 926, 166731. |
| [26] | Zhao, F. P.; Zhao, Y.; Wang, J.; Sun, Q.; Adair, K.; Zhang, S. M.; Luo, J.; Li, J. J.; Li, W. H.; Sun, Y. P.; Li, X. N.; Liang, J. W.; Wang, C. H.; Li, R. Y.; Huang, H.; Zhang, L.; Zhao, S. Q.; Lu, S. G.; Sun, X. L. Energy Storage Mater. 2020, 33, 139. |
| [27] | Jung, S. Y.; Rajagopal, R.; Ryu, K. S. J. Energy Chem. 2020, 47, 307. |
| [28] | Dietrich, C.; Weber, D. A.; Culver, S.; Senyshyn, A.; Sedlmaier, S. J.; Indris, S.; Janek, J.; Zeier, W. G. Inorg. Chem. 2017, 56, 6681. |
| [29] | Wu, J.; Liu, S.; Han, F.; Yao, X.; Wang, C. Adv. Mater. 2021, 33, 2000751. |
| [30] | Luo, S. T.; Wang, Z. Y.; Li, X. L.; Liu, X. Y.; Wang, H. D.; Ma, W. G.; Zhang, L. Q.; Zhu, L. Y.; Zhang, X. Nat. Commun. 2021, 12, 6968. |
| [31] | Han, F.; Westover, A. S.; Yue, J.; Fan, X.; Wang, F.; Chi, M.; Leonard, D. N.; Dudney, N.; Wang, H.; Wang, C. Nat. Energy 2019, 4, 187. |
| [32] | Jin, Y. M.; Liu, C. J.; Jia, Z. G.; Zong, X.; Li, D.; Fu, M. Y.; Wei, J. H.; Xiong, Y. P. J. Alloy Compd. 2021, 874, 159890. |
| [33] | Jia, Z. G.; Zhang, X. X.; Zhong, J.; Tian, H.; Xiong, Y. P. J. Electroanal. Chem. 2020, 856, 113659. |
/
| 〈 |
|
〉 |