

钯催化下杂芳基溴代物与偕二氟烯丙基硼试剂的交叉偶联反应
收稿日期: 2023-08-29
网络出版日期: 2023-10-24
基金资助
国家重点研发计划(2021YFF0701700); 国家自然科学基金(22101293); 先正达种业科技(中国)有限公司
Palladium-Catalyzed Cross-Coupling of Heteroaryl Bromides with gem-Difluoroallylborons
Received date: 2023-08-29
Online published: 2023-10-24
Supported by
National Key Research and Development Program of China(2021YFF0701700); National Natural Science Foundation of China(22101293); Syngenta Crop Protection AG
张大伟 , 赵海洋 , 冯笑甜 , 顾玉诚 , 张新刚 . 钯催化下杂芳基溴代物与偕二氟烯丙基硼试剂的交叉偶联反应[J]. 化学学报, 2024 , 82(2) : 105 -109 . DOI: 10.6023/A23080395
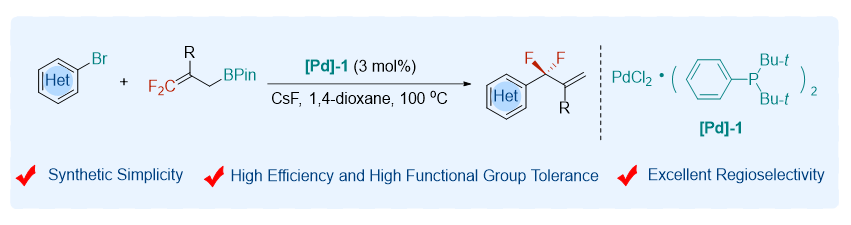
Due to the unique properties of fluorine atom(s), the introduction of fluorinated functional groups into molecules has become one of the powerful strategies in the discovery of new pharmaceuticals, agrochemicals, and advanced functional materials. Consequently, considerable efforts have been made to develop new and efficient methods for preparing organofluorine compounds. Among the fluorine functionalities, the gem-difluoroallyl group represents one of the attractive moieties due to the unique properties of the difluoromethylene group (CF2) and the synthetic versatility of the carbon-carbon double bond. Over the past decade, important progress has been made in the catalytic gem-difluoroallylation reactions. However, the efficient methods for the preparation of gem-difluoroallyl arenes remain limited despite their important applications in medicinal chemistry. Here, we report a palladium-catalyzed gem-difluoroallylation of heteroaryl bromides with gem-difluoroallylboronates. The reaction proceeds under mild conditions with high efficiency, high functional group tolerance, and excellent regioselectivity. A series of heteroaryl bromides are applicable to the reaction, providing facile access to gem-difluoroallyl heteroarenes of medicinal interest. A representative procedure for the palladium-catalyzed cross-coupling of heteroaryl bromides with gem-difluoroallylborons is as following: heteroaryl bromide (0.40 mmol, 1.0 equiv.) and (P(t-Bu)2Ph)2•PdCl2 (3.0 mol%) were added to a 25 mL of Schlenck tube. The tube was then evacuated and backfilled with Ar (3 times). CsF (2.0 equiv.), gem-difluoroallylboron (0.44 mmol, 1.1 equiv.), and 1,4-dioxane (2.0 mL) were added under Ar. The tube was screw capped and put into a preheated oil bath (100 ℃). After stirring for 2 h, the reaction mixture was cooled to room temperature and diluted with ethyl acetate (2.0 mL). The yield was determined by 19F NMR using fluorobenzene (1.0 equiv.) as an internal standard before working up. If necessary, the reaction mixture was diluted with EtOAc and filtered with a pad of cellite. The filtrate was concentrated, and the residue was purified with silica gel chromatography to give product 11.
| [1] | For selected reviews, see: (a) Hiyama, T. Organofluorine Compounds, Chemistry and Applications, Springer-Verlag, Berlin Heidelberg, 2000. |
| [1] | (b) Muller, K.; Faeh, C.; Diederich, F. Science 2007, 317, 1881. |
| [1] | (c) O'Hagan,D. Chem. Soc. Rev. 2008, 37, 308. |
| [1] | (d) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320. |
| [1] | (e) Huang, W. Y. Chin. J. Org. Chem. 1985, 5, 16. (in Chinese) |
| [1] | (黄维垣, 有机化学, 1985, 5, 16.) |
| [2] | (a) Müller, K.; Faeh, C.; Diederich, F. Science 2007, 317, 1881. |
| [2] | (b) Hagmann, W. K. J. Med. Chem. 2008, 51, 4359. |
| [2] | (c) Yang, Y.; You, Z.; Qing, F.-L. Acta Chim. Sinica 2012, 70, 2323. (in Chinese) |
| [2] | (杨义, 游正伟, 卿凤翎, 化学学报, 2012, 70, 2323.) |
| [2] | (d) O'Hagan, D.; Wang, Y.; Skibinski, M.; Slawin, A. M. Z. Pure Appl. Chem. 2012, 84, 1587. |
| [3] | For selected reviews, see: (a) Meanwell, N. A. J. Med. Chem. 2011, 54, 2529. |
| [3] | (b) Meanwell, N. A. J. Med. Chem. 2018, 61, 5822. |
| [4] | For selected examples, see: (a) Xue, F.; Li, H.; Delker, S. L.; Fang, J.; Martasek, P.; Roman, L. J.; Poulos, T. L.; Silverman, R. B. J. Am. Chem. Soc. 2010, 132, 14229. |
| [4] | (b) Anderson, M. O.; Zhang, J.; Liu, Y.; Yao, C.; Phuan, P.-W.; Verkman, A. S. J. Med. Chem. 2012, 55, 5942. |
| [4] | (c) Matthew, A. N.; Zephyr, J.; Hill, C. J.; Jahangir, M.; Newton, A.; Petropoulos, C. J.; Huang, W.; Kurt-Yilmaz, N.; Schiffer, C. A.; Ali, A. J. Med. Chem. 2017, 60, 5699. |
| [5] | (a) Markovsi, L. N.; Pahinnik, V. E.; Kirsanov, A. V. Synthesis 1973, 12, 787. |
| [5] | (b) Middleton, W. J. J. Org. Chem. 1975, 40, 574. |
| [6] | For selected reviews regarding fluorination and trifluoromethylation, see: (a) Furuya, T.; Kamlet, A. S.; Ritter, T. Nature 2011, 473, 470. |
| [6] | (b) Tomashenko, O. A.; Grushin, V. V. Chem. Rev. 2011, 111, 4475. |
| [7] | For transition-metal-catalyzed difluoroalkylation, see: (a) Feng, Z.; Xiao, Y.-L.; Zhang, X. Acc. Chem. Res. 2018, 51, 2264. |
| [7] | (b) An, L.; Tong, F.; Zhang, X. Acta Chim. Sinica 2018, 76, 977. (in Chinese) |
| [7] | (安伦, 童非非, 张新刚, 化学学报, 2018, 76, 977.) |
| [7] | (c) Wang, W.; Yu, Q.; Zhang, Q.; Li, J.; Hui, F.; Yang, J.; Lü, J. Chin. J. Org. Chem. 2018, 38, 1569. (in Chinese) |
| [7] | (王为强, 余秦伟, 张前, 李江伟, 惠丰, 杨建明, 吕剑, 有机化学, 2018, 38, 1569.) |
| [8] | (a) Ping, Y. Y.; Song, H. X.; Kong, W. Q. Chin. J. Org. Chem. 2022, 42, 3302 (in Chinese) |
| [8] | (平媛媛, 宋海霞, 孔望清, 有机化学, 2022, 42, 3302.) |
| [8] | (b) Gao, P.-P.; Xiao, W.-J.; Chen, J.-R. Chin. J. Org. Chem. 2022, 42, 3923. (in Chinese) |
| [8] | (高盼盼, 肖文精, 陈加荣, 有机化学, 2022, 42, 3923.) |
| [8] | (c) Link, J. O.; Taylor, J. G.; Xu, L.; Mitchell, M.; Guo, H.; Liu, H.; Kato, D.; Kirschberg, T.; Sun, J.; Squires, N.; Parrish, J.; Kellar, T.; Yang, Z. Y.; Yang, C.; Matles, M.; Wang, Y.; Wang, K.; Cheng, G.; Tian, Y.; Mogalian, E.; Mondou, E.; Cornpropst, M.; Perry, J.; Desai, M. C. J. Med. Chem. 2014, 57, 2033. |
| [8] | (d) Lamb, Y. N. Drugs 2017, 77, 1797. |
| [9] | Min, Q.-Q.; Yin, Z.; Feng, Z.; Guo, W.-H.; Zhang, X. J. Am. Chem. Soc. 2014, 136, 1230. |
| [10] | Tang, L.; Liu, Z.-Y.; She, W.; Feng, C. Chem. Sci. 2019, 10, 8701. |
| [11] | Lou, Y.-G.; Wang, A.-J.; Zhao, L.; He, L.-F.; Li, X.-F.; He, C.-Y.; Zhang, X. Chem. Commun. 2019, 55, 3705. |
| [12] | Sakamoto, S.; Butcher, T. W.; Yang, J. L.; Hartwig, J. F. Angew. Chem., Int. Ed. 2021, 60, 25746. |
| [13] | Ramachandran, P. C.; Tafelska-Kaczmarek, A.; Chatterjee, A. J. Org. Chem. 2012, 77, 9329. |
| [14] | (a) Kotha, S.; Behera, M.; Shah, V. R. Synlett 2005, 1877. |
| [14] | (b) Gerbino, D. C.; Mandolesi, S. D.; Schmalz, H.-G.; Podest, J. C. Eur. J. Org. Chem. 2009, 3964. |
| [14] | (c) Kotha, S.; Chavan, A. S.; Shaikh, M. J. Org. Chem. 2012, 77, 482. |
| [15] | (a) Surry, D. S.; Buchwald, S. L. Chem. Sci. 2011, 2, 57. |
| [15] | (b) Martin, R.; Buchwald, S. L. Acc. Chem. Res. 2008, 41, 1461. |
/
| 〈 |
|
〉 |