

基于二氟卡宾转化的芳基和烯基碘化物的三氟甲硫基化
收稿日期: 2023-08-19
网络出版日期: 2023-11-27
基金资助
国家重点研发计划(2021YFF0701700); 国家自然科学基金(21971252); 国家自然科学基金(21991122); 国家自然科学基金(22271181); 上海市科委(22ZR1423600)
Difluorocarbene-based Trifluoromethylthiolation of Aryl and Alkenyl Iodides
Received date: 2023-08-19
Online published: 2023-11-27
Supported by
National Key Research and Development Program of China(2021YFF0701700); National Natural Science Foundation of China(21971252); National Natural Science Foundation of China(21991122); National Natural Science Foundation of China(22271181); Science and Technology Commission of Shanghai Municipality(22ZR1423600)
郑剑 , 林锦鸿 , 肖吉昌 . 基于二氟卡宾转化的芳基和烯基碘化物的三氟甲硫基化[J]. 化学学报, 2024 , 82(2) : 115 -118 . DOI: 10.6023/A23080384
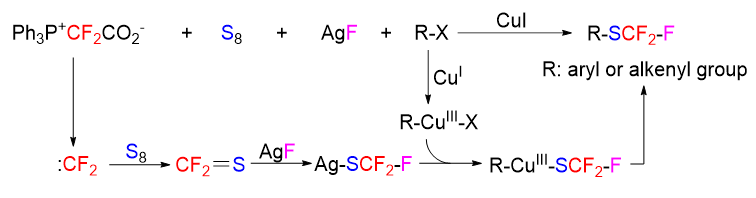
Difluorocarbene has found widespread applications in the synthesis of fluorine-containing molecules. We have previously found that difluorocarbene can react with elemental sulfur to produce thiocarbonyl fluoride, which is of great value for the new discoveries of difluorocarbene chemistry and the investigations of synthetic utilities of thiocarbonyl fluoride. We have developed the transformation of difluorocarbene into thiocarbonyl fluoride as a synthetic tool to achieve trifluoromethylthiolation of terminal alkynes and alkyl halides. In continuation of our research interest in this chemistry, herein we further apply the difluorocarbene transformation to the trifluoromethylthiolation of aryl and alkenyl iodides. Trifluoromethylthiolation is an active research area in organofluorine chemistry, and the commonly used trifluoromethylthiolation methods usually require the use of expensive CF3S-containing reagents. In contrast, in our protocol the CF3S group is generated in situ from difluorocarbene, elemental sulfur and a fluoride anion, all of which are cheap and easily available reagents. The general experimental procedure is shown as follows. Into a 5 mL sealed tube were added 4-phenyl phenyl iodide (1a, 56.0 mg, 0.2 mmol), S (57.8 mg, 1.8 mmol), Ph3P+CF2CO2– (PDFA) (213.8 mg, 0.6 mmol), AgF (0.5 mmol, 63.4 mg), ligand L1 (0.6 mmol, 158.9 mg), CuI (76.2 mg, 0.4 mmol), and dioxane (1.0 mL) under a N2 atmosphere. The reaction mixture was stirred at 110 ℃ for 8 h. After the reaction system was cooled to room temperature, Et3N (0.5 mL) was added to remove the excess elemental sulfur by a redox reaction (the final product would be contaminated by elemental sulfur if elemental sulfur was not removed). The mixture was diluted with 10 mL of saturated brine, and then the product was extracted with ethyl acetate. The combined organic layers were washed with brine, dried over sodium sulfate, and concentrated to 1 mL. The residue was subjected to flash column chromatography to afford the pure product.
| [1] | (a) Brahms, D.; Dailey, W. Chem. Rev. 1996, 96, 1585. |
| [1] | (b) Dilman, A. D.; Levin, V. V. Acc. Chem. Res. 2018, 51, 1272. |
| [1] | (c) Zhang, W.; Wang, Y. Tetrahedron Lett. 2018, 59, 1301. |
| [1] | (d) Zhou, W.; Pan, W.-J.; Chen, J.; Zhang, M.; Lin, J.-H.; Cao, W.; Xiao, J.-C. Chem. Commun. 2021, 57, 9316. |
| [1] | (e) Ma, X.; Su, J.; Song, Q. Acc. Chem. Res. 2023, 56, 592. |
| [2] | (a) Park, J. D.; Benning, A. F.; Downing, F. B.; Laucius, J. F.; McHarness, R. C. Ind. Eng. Chem. 1947, 39, 354. |
| [2] | (b) Hu, M.; Ni, C.; Li, L.; Han, Y.; Hu, J. J. Am. Chem. Soc. 2015, 137, 14496. |
| [2] | (c) Zheng, J.; Lin, J.-H.; Yu, L.-Y.; Wei, Y.; Zheng, X.; Xiao, J.-C. Org. Lett. 2015, 17, 6150. |
| [2] | (d) Zhang, Z.; Yu, W.; Wu, C.; Wang, C.; Zhang, Y.; Wang, J. Angew. Chem., Int. Ed. 2016, 55, 273. |
| [2] | (e) Li, L.; Ni, C.; Xie, Q.; Hu, M.; Wang, F.; Hu, J. Angew. Chem., Int. Ed. 2017, 56, 9971. |
| [3] | (a) Li, L.; Wang, F.; Ni, C.; Hu, J. Angew. Chem., Int. Ed. 2013, 52, 12390. |
| [3] | (b) Deng, X.-Y.; Lin, J.-H.; Zheng, J.; Xiao, J.-C. Chem. Commun. 2015, 51, 8805. |
| [3] | (c) Xie, Q.; Ni, C.; Zhang, R.; Li, L.; Rong, J.; Hu, J. Angew. Chem., Int. Ed. 2017, 56, 3206. |
| [3] | (d) Sap, J. B. I.; Meyer, C. F.; Ford, J.; Straathof, N. J. W.; Durr, A. B.; Lelos, M. J.; Paisey, S. J.; Mollner, T. A.; Hell, S. M.; Trabanco, A. A.; Genicot, C.; am Ende, C. W.; Paton, R. S.; Tredwell, M.; Gouverneur, V. Nature 2022, 606, 102. |
| [4] | (a) Fujioka, Y.; Amii, H. Org. Lett. 2008, 10, 769. |
| [4] | (b) Wang, F.; Luo, T.; Hu, J.; Wang, Y.; Krishnan, H.; Jog, P.; Ganesh, S.; Prakash, G.; Olah, G. Angew. Chem., Int. Ed. 2011, 50, 7153. |
| [4] | (c) Wang, F.; Zhang, W.; Zhu, J.; Li, H.; Huang, K.-W.; Hu, J. Chem. Commun. 2011, 47, 2411. |
| [4] | (d) Rulliere, P.; Cyr, P.; Charette, A. B. Org. Lett. 2016, 18, 1988. |
| [4] | (e) Garcia-Dominguez, A.; West, T. H.; Primozic, J. J.; Grant, K. M.; Johnston, C. P.; Cumming, G. G.; Leach, A. G.; Lloyd-Jones, G. C. J. Am. Chem. Soc. 2020, 142, 14649. |
| [5] | (a) Brothers, P. J.; Roper, W. R. Chem. Rev. 1988, 88, 1293. |
| [5] | (b) Harrison, D. J.; Lee, G. M.; Leclerc, M. C.; Korobkov, I.; Baker, R. T. J. Am. Chem. Soc. 2013, 135, 18296. |
| [5] | (c) Deng, X.-Y.; Lin, J.-H.; Xiao, J.-C. Org. Lett. 2016, 18, 4384. |
| [5] | (d) Feng, Z.; Min, Q.-Q.; Zhang, X. Org. Lett. 2016, 18, 44. |
| [5] | (e) Feng, Z.; Min, Q. Q.; Fu, X. P.; An, L.; Zhang, X. Nat. Chem. 2017, 9, 918. |
| [5] | (f) Fu, X.-P.; Xue, X.-S.; Zhang, X.-Y.; Xiao, Y.-L.; Zhang, S.; Guo, Y.-L.; Leng, X.; Houk, K. N.; Zhang, X. Nat. Chem. 2019, 11, 948. |
| [5] | (g) Xu, Z.-W.; Zhang, W.; Lin, J.-H.; Jin, C. M.; Xiao, J.-C. Chin. J. Chem. 2020, 38, 1647. |
| [5] | (h) Zeng, X.; Li, Y.; Min, Q.-Q.; Xue, X.-S.; Zhang, X. Nat. Chem. 2023. |
| [5] | (i) Zhang, X.-Y.; Sun, S.-P.; Sang, Y.-Q.; Xue, X.-S.; Min, Q.-Q.; Zhang, X. Angew. Chem., Int. Ed. 2023, e202306501. |
| [6] | (a) Kim, Y.; Heo, J.; Kim, D.; Chang, S.; Seo, S. Nat. Commun. 2020, 11, 4761. |
| [6] | (b) Su, J.; Ma, X.; Ou, Z.; Song, Q. ACS Cent. Sci. 2020, 6, 1819. |
| [6] | (c) Liu, X.; Sheng, H.; Zhou, Y.; Song, Q. Org. Lett. 2021, 23, 2543. |
| [6] | (d) Liu, A.; Ni, C.; Xie, Q.; Hu, J. Angew. Chem., Int. Ed. 2022, 61, e202115467. |
| [6] | (e) Wang, S.; Li, X.; Jin, S.; Liu, K.; Dong, C.; Su, J.; Song, Q. Org. Chem. Front. 2022, 9, 1282. |
| [6] | (f) Zhang, G.; Shi, Q.; Hou, M.; Yang, K.; Wang, S.; Wang, S.; Li, W.; Li, C.; Qiu, J.; Xu, H.; Zhou, L.; Wang, C.; Li, S.-J.; Lan, Y.; Song, Q. CCS Chem. 2022, 4, 1671. |
| [6] | (g) Chen, S.; Huang, H.; Li, X.; Ma, X.; Su, J.; Song, Q. Org. Lett. 2023, 25, 1178. |
| [7] | (a) Ma, X.; Zhou, Y.; Song, Q. Org. Lett. 2018, 20, 4777. |
| [7] | (b) Ma, X.; Mai, S.; Zhou, Y.; Cheng, G.-J.; Song, Q. Chem. Commun. 2018, 54, 8960. |
| [7] | (c) Si, Y.-X.; Zhu, P.-F.; Zhang, S.-L. Org. Lett. 2020, 22, 9086. |
| [7] | (d) Jiang, B.-J.; Zhang, S.-L. Adv. Synth. Catal. 2022, 364, 2157. |
| [8] | (a) Zheng, J.; Cai, J.; Lin, J.-H.; Guo, Y.; Xiao, J.-C. Chem. Commun. 2013, 49, 7513. |
| [8] | (b) Zheng, J.; Lin, J.-H.; Cai, J.; Xiao, J.-C. Chem.-Eur. J. 2013, 19, 15261. |
| [8] | (c) Lin, J.-H.; Xiao, J.-C. Acc. Chem. Res. 2020, 53, 1498. |
| [9] | (a) Qiao, Y.; Si, T.; Yang, M.-H.; Altman, R. A. J. Org. Chem. 2014, 79, 7122. |
| [9] | (b) Liu, Y.; Zhang, K.; Huang, Y.; Pan, S.; Liu, X.-Q.; Yang, Y.; Jiang, Y.; Xu, X.-H. Chem. Commun. 2016, 52, 5969. |
| [9] | (c) Panferova, L. I.; Tsymbal, A. V.; Levin, V. V.; Struchkova, M. I.; Dilman, A. D. Org. Lett. 2016, 18, 996. |
| [9] | (d) Panferova, L. I.; Levin, V. V.; Struchkova, M. I.; Dilman, A. D. Chem. Commun. 2019, 55, 1314. |
| [9] | (e) Ilin, E. A.; Smirnov, V. O.; Volodin, A. D.; Korlyukov, A. A.; Dilman, A. D. Chem. Commun. 2020, 56, 7140. |
| [9] | (f) Kee, C. W.; Tack, O.; Guibbal, F.; Wilson, T. C.; Isenegger, P. G.; Imiolek, M.; Verhoog, S.; Tilby, M.; Boscutti, G.; Ashworth, S.; Chupin, J.; Kashani, R.; Poh, A. W. J.; Sosabowski, J. K.; Macholl, S.; Plisson, C.; Cornelissen, B.; Willis, M. C.; Passchier, J.; Davis, B. G.; Gouverneur, V. J. Am. Chem. Soc. 2020, 142, 1180. |
| [9] | (g) Jia, Y.; Yuan, Y.; Huang, J.; Jiang, Z.-X.; Yang, Z. Org. Lett. 2021, 23, 2670. |
| [10] | (a) Zheng, J.; Wang, L.; Lin, J.-H.; Xiao, J.-C.; Liang, S. H. Angew. Chem., Int. Ed. 2015, 54, 13236. |
| [10] | (b) Zheng, J.; Cheng, R.; Lin, J.-H.; Yu, D. H.; Ma, L.; Jia, L.; Zhang, L.; Wang, L.; Xiao, J.-C.; Liang, S. H. Angew. Chem., Int. Ed. 2017, 56, 3196. |
| [10] | (c) Yu, J.; Lin, J.-H.; Xiao, J.-C. Angew. Chem., Int. Ed. 2017, 56, 16669. |
| [11] | Yu, J.; Lin, J.-H.; Yu, D.; Du, R.; Xiao, J.-C. Nat. Commun. 2019, 10, 5362. |
| [12] | Zhang, M.; Lin, J.-H.; Xiao, J.-C. Angew. Chem., Int. Ed. 2019, 58, 6079. |
| [13] | (a) Middleton, W. J.; Howard, E. G.; Sharkey, W. H. J. Am. Chem. Soc. 1961, 83, 2589. |
| [13] | (b) Middleton, W. J.; Howard, E. G.; Sharkey, W. H. J. Org. Chem. 1965, 30, 1375. |
| [13] | (c) Eschwey, M.; Sundermeyer, W.; Stephenson, D. S. Chem. Ber. 1983, 116, 1623. |
| [13] | (d) Waterfeld, A. Chem. Ber. 1990, 123, 1635. |
| [14] | (a) Luo, J.-J.; Zhang, M.; Lin, J.-H.; Xiao, J.-C. J. Org. Chem. 2017, 82, 11206. |
| [14] | (b) Liu, Z.; Long, J.; Xiao, X.; Lin, J.-H.; Zheng, X.; Xiao, J.-C.; Cao, Y.-C. Chin. Chem. Lett. 2019, 30, 714. |
| [14] | (c) He, G.; Jiang, Y.-H.; Xiao, X.; Lin, J.-H.; Zheng, X.; Du, R.-B.; Cao, Y.-C.; Xiao, J.-C. J. Fluorine Chem. 2020, 230, 109437. |
| [15] | (a) Leo, A.; Hansch, C.; Elkins, D. Chem. Rev. 1971, 71, 525. |
| [15] | (b) Hansch, C.; Leo, A.; Unger, S. H.; Kim, K. H.; Nikaitani, D.; Lien, E. J. J. Med. Chem. 1973, 16, 1207. |
| [15] | (c) Hansch, C.; Leo, A.; Taft, R. Chem. Rev. 1991, 91, 165. |
| [16] | Xu, X.-H.; Matsuzaki, K.; Shibata, N. Chem. Rev. 2015, 115, 731. |
| [17] | (a) Chu, L.; Qing, F.-L. Acc. Chem. Res. 2014, 47, 1513. |
| [17] | (b) Shao, X.; Xu, C.; Lu, L.; Shen, Q. Acc. Chem. Res. 2015, 48, 1227. |
| [17] | (c) Xu, C.; Wang, S.; Shen, Q. ACS Sustainable Chem. Eng. 2022, 10, 6889. |
| [17] | (d) Shen, Q. J. Org. Chem. 2023, 88, 3359. |
| [17] | (e) Qing, F.-L.; Liu, X.-Y.; Ma, J.-A.; Shen, Q.; Song, Q.; Tang, P. CCS Chem. 2022, 4, 2518. |
/
| 〈 |
|
〉 |