

磷酸氧钒钠纳米片的可控制备研究
收稿日期: 2023-10-14
网络出版日期: 2024-01-05
Research on Preparation of Nano-flake Sodium Vanadyl Phosphate
Received date: 2023-10-14
Online published: 2024-01-05
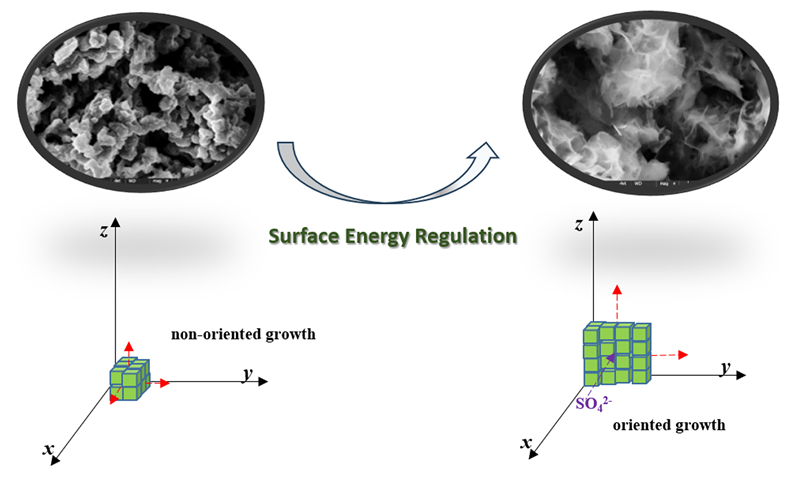
基于磷酸氧钒钠的制备条件苛刻、颗粒粒径大和电导率差等问题, 提出了一种制备纳米片状磷酸氧钒钠的新方法. 通过特定条件下的表面能调控技术, 实现纳米片状磷酸氧钒钠的可控制备. 通过对不同生长时间的产物进行监测分析, 研究了纳米片的形成过程. 结合密度泛函理论(DFT)模拟计算, 分析了片状磷酸氧钒钠的成片机理. 调控不同硫酸根的添加量, 研究了不同硫酸根条件下磷酸氧钒钠的微观形貌变化规律, 验证了片状磷酸氧钒钠的成片机制. 研究结果表明: $\text{SO}_{\text{4}}^{2}$离子在不同晶面的吸附改变了不同晶面的表面能大小, 改变了磷酸氧钒钠晶核优势生长取向, 实现了定向生长与自组装, 最终获得了独特的纳米片状磷酸氧钒钠. 由于独特的纳米片状微观形貌, 所制备的片状磷酸氧钒钠具有更高的比表面积和压实密度. 其比表面积为16.2 m2/g, 优于纳米颗粒状磷酸氧钒钠的4.3 m2/g, 压实密度达到1.86 g/cm3, 高于颗粒状磷酸氧钒钠的1.77 g/cm3. 此外, 纳米片状磷酸氧钒钠还表现出优异的储钠性能. 在 0.1 C电流密度条件下, 纳米片状磷酸氧钒钠的放电比容量达到93.76 mAh/g, 大于相同放电电流密度下, 纳米颗粒状磷酸氧钒钠的82.85 mAh/g. 同时, 10 C电流密度条件下, 循环100次后, 仍能保持105%的初始容量(相同情况下, 纳米颗粒状磷酸氧钒钠仅有92%), 表现出良好的循环稳定性.
张东彬 , 袁欣然 , 辛亚男 , 刘天豪 , 韩慧果 , 杜光超 , 滕艾均 . 磷酸氧钒钠纳米片的可控制备研究[J]. 化学学报, 2024 , 82(3) : 274 -280 . DOI: 10.6023/A23100449
Based on the severe preparation conditions, large particle size and poor conductivity, a new method to prepare nano-flake sodium vanadyl phosphate is proposed. By the method of surface energy control technique under specific conditionsion, the controllable preparation of nano-flake sodium vanadyl phosphate was achieved. Through monitoring and analysis of the products under the different growth times, the formation process of nano-flake was studied, and the formation mechanism of sodium vanadyl phosphate was analyzed by density functional theory (DFT) simulation. The microstructure of sodium vanadyl phosphate is studied by adjusting the amount of sulfate radical, to verify the formation mechanism of unique nano-flake sodium vanadyl phosphate. The results show that the adsorption of $\text{SO}_{\text{4}}^{2}$ ions on different crystal faces changes the surface energy of different crystal faces, changes the dominant growth orientation of crystal nuclei, realizes directional growth and self-assembly, and finally gets the unique nano-flake sodium vanadyl phosphate. Because of the unique nano-flake micro-morphology, the prepared nano-flake sodium vanadyl phosphate has higher specific surface area and compaction density. The specific surface area of nano-flake sodium vanadyl phosphate is 16.2 m2/g, and the compaction density is 1.86 g/cm3, which is higher than that of the nano-particle sodium vanadyl phosphate, while 4.3 m2/g for the specific surface area and 1.77 g/cm3 for the compaction density of nano-particle sodium vanadyl phosphate. In addition, nano-flake sodium vanadyl phosphate also shows excellent sodium storage properties. Under the condition of current density at 0.1 C, the discharge specific capacity of nano-flake sodium vanadate phosphate reaches 93.76 mAh/g, which is larger than that of nano- particle sodium vanadyl phosphate (82.85 mAh/g) at the same discharge current density. At the same time, after 100 charge and discharge cycles at 10 C, 105% of the initial capacity can be maintained (in the same condition, only 92% of the initial capacity can be maintained for the nano-particle sodium vanadyl phosphate), showing good cycle stability.

| [1] | Chen, G. X.; Huang, Q.; Wu, T.; Lu, L. Adv. Funct. Mater. 2020, 2001289. |
| [2] | Zeng, X. G.; Peng, J.; Guo, Y.; Zhu, H. F.; Huang, X. Front. Chem. 2020, 8, 635. |
| [3] | Sun, C.; Deng, Z. R.; Jiang, N. B.; Zhang, L. L.; Fang, H.; Yang, X. L. Energy Storage Science and Technology 2022, 11, 1184 (in Chinese). |
| [3] | (孙畅, 邓泽荣, 江宁波, 张露露, Fang Hui, 杨学林, 储能科学与技术, 2022, 11, 1184.) |
| [4] | Liu, X. H.; Feng, G. L.; Wu, Z. G.; Wang, D.; Wu, C.; Yang, L.; Xiang, W.; Chen, Y. X.; Guo, X. D.; Zhong, B. H. J. Alloy. Compd. 2019, 815, 152430. |
| [5] | Jian, Z. L.; Zhao, L.; Pan, H. L.; Hu, Y. S.; Li, H.; Chen, W.; Chen, L. Q. Electrochem. Commun. 2012, 14, 86. |
| [6] | Shen, X.; Zhou, Q.; Han, M.; Qi, X. G.; Li, B.; Zhang, Q. Q.; Zhao, J. M.; Yang, C.; Liu, H. Z.; Hu, Y.-S. Nat. Commun. 2021, 12, 2848. |
| [7] | Yang, J.; Han, D.-W.; Jo, M. R.; Song, K.; Kim, Y.-I.; Chou, S.-L.; Liu, H.-K.; Kang, Y.-M. J. Mater. Chem. A 2015, 3, 1005. |
| [8] | Liu, Q.; Wang, D. X.; Yang, X.; Chen, N.; Wang, C. Z.; Bie, X. F.; Wei, Y. J.; Chen, G.; Du, F. J. Mater. Chem. A 2015, 3, 21478. |
| [9] | Wang, P. Y.; Zhang, X. L.; Xu, J. Q. New Chemical Materials 2007, 35, 14 (in Chinese). |
| [9] | (王培义, 张晓丽, 徐甲强, 化工新型材料, 2007, 35, 14.) |
| [10] | Xu, W. J.; Jia, J.; Wang, T.; Li, C.; He, B. W.; Zong, J. P.; Wang, Y. W.; Fan, H. J.; Xu, H. X.; Feng, Y. H.; Chen, H. Y. Angew. Chem., Int. Ed. 2020, 59, 22246. |
| [11] | Jia, J.; Liu, G. Y.; Xu, W. J.; Tian, X. L.; Li, S. B.; Han, F.; Feng, Y. H.; Dong, X. C.; Chen, H. Y. Angew. Chem., Int. Ed. 2020, 59, 14443. |
| [12] | Chen, T.; Yang, Y.; Zhao, W. Y.; Pan, D. Q.; Zhu, C. T.; Lin, F. Y.; Guo, X. Y. Acta Chim. Sinica 2019, 77, 447 (in Chinese). |
| [12] | (陈甜, 杨英, 赵婉玉, 潘德群, 朱从潭, 林飞宇, 郭学益, 化学学报, 2019, 77, 447.) |
| [13] | Wang, H. X.; Yang, G.; Cheng, T. S.; Wang, N.; Sun, R.; Wong, C.-P. Acta Chim. Sinica 2019, 77, 316 (in Chinese). |
| [13] | (王海旭, 杨光, 程天舒, 王宁, 孙蓉, 汪正平, 化学学报, 2019, 77, 316.) |
| [14] | Zhang, G. X.; Chen, Y. M.; He, Z.-N.; Lin, C.; Chen, Y.-G.; Guo, H.-B. Journal of Inorganic Materials 2018, 3, 289 (in Chinese). |
| [14] | (张国雄, 陈月梅, 何臻妮, 林川, 陈益钢, 郭海波, 无机材料学报, 2018, 3, 289.) |
| [15] | Fang, Y. J.; Liu, Q.; Xiao, L. F.; Rong, Y. C.; Liu, Y. D.; Chen, Z. X.; Ai, X. P.; Cao, Y. L.; Yang, H. X.; Xie, J.; Sun, C. J.; Zhang, X. Y.; Aoun, B.; Xing, X. R.; Xiao, X. H.; Ren, Y. Chem 2018, 4, 1. |
| [16] | Shen, X.; Han, M.; Li, X. W.; Zhang, P.; Yang, C.; Liu, H. Z.; Hu, Y.-S. ACS Appl. Mater. Interfaces 2022, 14, 6841. |
| [17] | Qi, Y. R.; Tong, Z. Z.; Zhao, J. M.; Ma, L.; Wu, T. P.; Liu, H. Z.; Yang, C.; Lu, J.; Hu, Y.-S. Joule. 2018, 2, 1. |
| [18] | Zhang, D. B.; Kong, X. G.; Jiang, M. H.; Lei, D. Q.; Lei, X. D. ACS Sustainable Chem. Eng. 2019, 7, 4420. |
/
| 〈 |
|
〉 |