

可见光介导炔烃的自由基1,2-官能团化反应新进展
收稿日期: 2024-03-18
网络出版日期: 2024-04-29
基金资助
项目受国家自然科学基金(22071062); 项目受国家自然科学基金(22271096); 中央高校基本科研业务费专项资金(2022ZYGXZR107)
Recent Advances in Visible Light Induced Radical 1,2-Functionalization of Alkynes
Received date: 2024-03-18
Online published: 2024-04-29
Supported by
National Natural Science Foundation of China(22071062); National Natural Science Foundation of China(22271096); Fundamental Research Funds for the Central Universities, SCUT(2022ZYGXZR107)
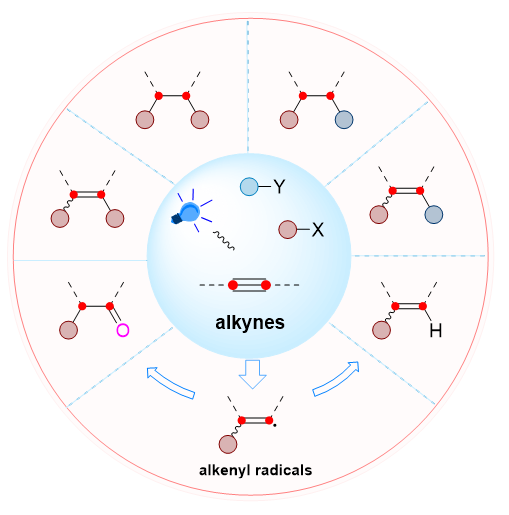
作为有机分子中最基本的官能团之一, 包含两个π键的碳碳三键蕴藏着多样有机转化的巨大潜力. 其中, 基于自由基对炔烃加成的官能团化反应是合成多样的功能化烯烃、酮, 乃至烷烃分子的有效途径, 同时也是构建多个化学键的重要手段. 近十几年来, 由于可见光介导的自由基反应策略符合绿色化学和可持续发展的理念, 使得这种策略在炔烃官能团化方面的应用受到了广泛的关注. 时至今日, 光介导炔烃的自由基官能团化反应得到了空前的发展, 随之涌现出许多新方法. 本文主要综述了近五年来可见光介导炔烃的自由基1,2-官能团化反应的新进展, 以构建的化学键种类进行分类, 对涉及的自由基种类和反应类型进行了系统总结. 分析讨论了反应的机理、普适性以及优势与不足, 并对该领域所面临的挑战和机遇进行了展望.
李康葵 , 龙先扬 , 黄岳 , 祝诗发 . 可见光介导炔烃的自由基1,2-官能团化反应新进展[J]. 化学学报, 2024 , 82(6) : 658 -676 . DOI: 10.6023/A24030090
As one of the most basic functional groups in organic molecules, the carbon-carbon triple bond containing two π-bonds harbor great potential for diverse organic transformations. Among them, the functionalization reaction based on the addition of free radicals to alkynes is an effective way to synthesize diverse functionalized olefins, ketones, and even alkane molecules, as well as an important tool for constructing multiple chemical bonds simultaneously. In the last decade, the application of visible light-induced radical reaction strategy for alkyne functionalization has received widespread attention due to it align with the concepts of green chemistry and sustainable development. Nowadays, photoinduced radical functionalization of alkynes has been developed unprecedentically, and many new methods have emerged. Indeed, light-induced radical functionalization reactions of alkynes provide a relatively green approach to the diverse transformations of alkynes. These conversions include hydrofunctionalization, oxidative functionalization, difunctionalization, and so on. These reactions feature mild conditions and easy operation, and do not even require the addition of metals and photosensitizers. Importantly, they provide an efficient way for the construction of valuable skeletons. In particular, the difunctionalization reaction enables the synthesis of high-added-value challenging molecules from simple feedstocks. In these reactions, the alkenyl radicals formed by radical addition to alkynes are the key intermediates. Thus, the type of radical precursor and the further transformation of the alkenyl radical determine the type of chemical bond constructed and the structure of the final product. This paper mainly reviews the new advances in photoinduced radical 1,2-functionalization reactions of alkynes in the past five years, classifying them in terms of the types of chemical bonds constructed, and providing a systematic summary of the types of radicals and reactions. The mechanism and universality of the reactions, as well as its advantages and disadvantages were discussed, and the challenges and opportunities facing the field were outlooked as well.

Key words: visible light; alkynes; radical reaction; functionalization; photocatalysis
| [1] | (a) Gleiter, R.; Werz, D. B. Chem. Rev. 2010, 110, 4447. |
| [1] | (b) Brand, J. P.; Waser, J. Chem. Soc. Rev. 2012, 41, 4165. |
| [1] | (c) Bhunia, S.; Ghosh, P.; Patra, S. R. Adv. Synth. Catal. 2020, 362, 3664. |
| [1] | (d) Heravi, M. M.; Dehghani, M.; Zadsirjan, V.; Ghanbarian, M. Curr. Org. Synth. 2019, 16, 205. |
| [1] | (e) Chen, L.; Chen, K.; Zhu, S. Chem 2018, 4, 1208. |
| [1] | (f) Patel, M.; Saunthwal, R. K.; Verma, A. K. Acc. Chem. Res. 2017, 50, 240. |
| [1] | (g) Alabugin, I. V.; Gonzalez-Rodriguez, E. Acc. Chem. Res. 2018, 51, 1206. |
| [1] | (h) Fang, G.; Bi, X. Chem. Soc. Rev. 2015, 44, 8124. |
| [1] | (i) Khan, R.; Chen, J.; Fan, B. Adv. Synth. Catal. 2020, 362, 1564. |
| [2] | (a) Wille, U. Chem. Rev. 2013, 113, 813. |
| [2] | (b) Hu, C.; Mena, J.; Alabugin, I. V. Nat. Rev. Chem. 2023, 7, 405. |
| [2] | (c) Ren, X.; Lu, Z. Chin. J. Catal. 2019, 40, 1003. |
| [2] | (d) Chalotra, N.; Kumar, J.; Naqvi, T.; Shah, B. A. Chem. Commun. 2021, 57, 11285. |
| [2] | (e) Bag, D.; Kour, H.; Sawant, S. D. Org. Biomol. Chem. 2020, 18, 8278. |
| [3] | (a) Zhang, Y.; Cai, Z.; Warratz, S.; Ma, C.; Ackermann, L. Sci. China Chem. 2023, 66, 703. |
| [3] | (b) Deng, W.; Li, Y.; Li, Y.-G.; Bao, H. Synthesis 2018, 50, 2974. |
| [3] | (c) Ma, J.; Li, J.; Meng, Q.; Zeng, X. Chin. J. Org. Chem. 2023, 43, 2040. (in Chinese) |
| [3] | (马佳敏, 李姣兄, 孟千森, 曾祥华, 有机化学, 2023, 43, 2040.) |
| [4] | (a) Romero, N. A.; Nicewicz, D. A. Chem. Rev. 2016, 116, 10075. |
| [4] | (b) Prier, C. K.; Rankic, D. A.; MacMillan, D. W. C. Chem. Rev. 2013, 113, 5322. |
| [4] | (c) Milligan, J. A.; Phelan, J. P.; Badir, S. O.; Molander, G. A. Angew. Chem. Int. Ed. 2019, 58, 6152. |
| [4] | (d) Gao, P.-P.; Xiao, W.-J.; Chen, J.-R. Chin. J. Org. Chem. 2022, 42, 3923. (in Chinese) |
| [4] | (高盼盼, 肖文精, 陈加荣, 有机化学, 2022, 42, 3923.) |
| [4] | (e) Wang, H.; Wu, P.; Zhao, X.; Zeng, J.; Wan, Q. Acta Chim. Sinica 2019, 77, 231. (in Chinese) |
| [4] | (王浩, 吴品儒, 赵祥, 曾静, 万谦, 化学学报, 2019, 77, 231.) |
| [4] | (f) Laishram, R. D.; Chen, J.; Fan, B. Chem. Rec. 2021, 21, 69. |
| [4] | (g) Zhang, J.; Chen, Y. Acta Chim. Sinica 2017, 75, 41. (in Chinese) |
| [4] | (张晶, 陈以昀, 化学学报, 2017, 75, 41.) |
| [4] | (h) Wang, D.; Zhang, L.; Luo, S. Acta Chim. Sinica 2017, 75, 22. (in Chinese) |
| [4] | (王德红, 张龙, 罗三中, 化学学报, 2017, 75, 22.) |
| [5] | Giese, B.; Lachhein, S. Angew. Chem., Int. Ed. Engl. 1982, 21, 768. |
| [6] | (a) Zheng, H.; Su, J.; Zhou, Y.; Zhu, G. Chin. J. Org. Chem. 2022, 42, 4060. (in Chinese) |
| [6] | (郑汉良, 苏静雯, 周雨露, 朱钢国, 有机化学, 2022, 42, 4060.) |
| [6] | (b) Liao, J.; Yang, X.; Ouyang, L.; Lai, Y.; Huang, J.; Luo, R. Org. Chem. Front. 2021, 8, 1345. |
| [6] | (c) Zhou, Y.; Gu, Z.; Hong, Y.; Chen, H.; Luo, J.; Zheng, H.; Zhu, G. Org. Chem. Front. 2024, 11, 1232. |
| [7] | Ren, Y.-Y.; Zheng, X.; Zhang, X. Synlett 2018, 29, 1028. |
| [8] | Meyer, C. F.; Hell, S. M.; Misale, A.; Trabanco, A. A.; Gouverneur, V. Angew. Chem. Int. Ed. 2019, 58, 8829. |
| [9] | Li, K.; Zhang, X.; Chen, J.; Gao, Y.; Yang, C.; Zhang, K.; Zhou, Y.; Fan, B. Org. Lett. 2019, 21, 9914. |
| [10] | Deng, H.-P.; Fan, X.-Z.; Chen, Z.-H.; Xu, Q.-H.; Wu, J. J. Am. Chem. Soc. 2017, 139, 13579. |
| [11] | Song, Z.-Q.; Liu, Z.; Gan, Q.-C.; Lei, T.; Tung, C.-H.; Wu, L.-Z. Org. Lett. 2020, 22, 832. |
| [12] | Liu, F.; Zhang, K.; Zhao, X.-F.; Meng, Q.-X.; Zhao, T.-S.; Tian, W.-F.; He, Y.-Q. Tetrahedron Lett. 2023, 115, 154321. |
| [13] | Yue, H.; Zhu, C.; Kancherla, R.; Liu, F.; Rueping, M. Angew. Chem. Int. Ed. 2020, 59, 5738. |
| [14] | Lai, S.-Z.; Yang, Y.-M.; Xu, H.; Tang, Z.-Y.; Luo, Z. J. Org. Chem. 2020, 85, 15638. |
| [15] | Wang, H.; Li, Y.; Tang, Z.; Wang, S.; Zhang, H.; Cong, H.; Lei, A. ACS Catal. 2018, 8, 10599. |
| [16] | Huang, T.; Saga, Y.; Guo, H.; Yoshimura, A.; Ogawa, A.; Han, L.-B. J. Org. Chem. 2018, 83, 8743. |
| [17] | Ikeshita, D.; Masuda, Y.; Ishida, N.; Murakami, M. Org. Lett. 2022, 24, 2504. |
| [18] | Kaur, S.; Zhao, G.; Busch, E.; Wang, T. Org. Biomol. Chem. 2019, 17, 1955. |
| [19] | Burykina, J. V.; Shlapakov, N. S.; Gordeev, E. G.; K?nig, B.; Ananikov, V. P. Chem. Sci. 2020, 11, 10061. |
| [20] | Wu, X.; Gao, B. Org. Lett. 2023, 25, 8722. |
| [21] | Zhong, M.; Gagné, Y.; Hope, T. O.; Pannecoucke, X.; Frenette, M.; Jubault, P.; Poisson, T. Angew. Chem. Int. Ed. 2021, 60, 14498. |
| [22] | Tang, H.; Zhang, M.; Zhang, Y.; Luo, P.; Ravelli, D.; Wu, J. J. Am. Chem. Soc. 2023, 145, 5846. |
| [23] | Huo, L.; Li, X.; Zhao, Y.; Li, L.; Chu, L. J. Am. Chem. Soc. 2023, 145, 9876. |
| [24] | Malpani, Y. R.; Biswas, B. K.; Han, H. S.; Jung, Y.-S.; Han, S. B. Org. Lett. 2018, 20, 1693. |
| [25] | Lv, Y.; Liu, Q.; Liu, F.; Yue, H.; Li, J.-S.; Wei, W. Tetrahedron Lett. 2020, 61, 151335. |
| [26] | Hosseini-Sarvari, M.; Valikhani, A. New J. Chem. 2021, 45, 12464. |
| [27] | Song, T.; Zhang, Y.; Wang, C.; Li, Y.; Yang, Y. Chin. J. Chem. 2022, 40, 2618. |
| [28] | Liu, L.; Liu, M.; Liu, B.; Wang, Q.; Li, Y.; Feng, K.; Qiu, R.; Zhou, Y. Tetrahedron Lett. 2023, 133, 154823. |
| [29] | (a) Badir, S. O.; Molander, G. A. Chem 2020, 6, 1327. |
| [29] | (b) Zhu, C.; Yue, H.; Chu, L.; Rueping, M. Chem. Sci. 2020, 11, 4051. |
| [29] | (c) Zhu, S.; Zhao, X.; Li, H.; Chu, L. Chem. Soc. Rev. 2021, 50, 10836. |
| [29] | (d) Dong, Y.-J.; Zhu, B.; Geng, Y.; Zhao, Z.-W.; Su, Z.-M.; Guan, W. CCS Chem. 2021, 4, 1429. |
| [29] | (e) Xu, L.; Wang, F.; Chen, F.; Zhu, S.; Chu, L. Chin. J. Org. Chem. 2022, 42, 1. (in Chinese) |
| [29] | (徐磊, 王方, 陈凡, 朱圣卿, 储玲玲, 有机化学, 2022, 42, 1.) |
| [30] | Guo, L.; Song, F.; Zhu, S.; Li, H.; Chu, L. Nat. Commun. 2018, 9, 4543. |
| [31] | Qin, J.; Zhang, Z.; Lu, Y.; Zhu, S.; Chu, L. Chem. Sci. 2023, 14, 12143. |
| [32] | Yang, B.; Li, S.-J.; Wang, Y.; Lan, Y.; Zhu, S. Nat. Commun. 2021, 12, 5257. |
| [33] | Yang, B.; Lu, S.; Wang, Y.; Zhu, S. Nat. Commun. 2022, 13, 1858. |
| [34] | Zhang, Y.; Han, Y.; Zhu, S.; Qing, F.-L.; Xue, X.-S.; Chu, L. Angew. Chem. Int. Ed. 2022, 61, e202210838. |
| [35] | Wang, J.; Wu, X.; Cao, Z.; Zhang, X.; Wang, X.; Li, J.; Zhu, C. Adv. Sci. 2024, 2309022. |
| [36] | Lee, W.; Lee, Y.; Yoo, M.; Han, S. B.; Kim, H. J. Org. Chem. Front. 2020, 7, 3209. |
| [37] | Dong, X.; Jiang, W.; Hua, D.; Wang, X.; Xu, L.; Wu, X. Chem. Sci. 2021, 12, 11762. |
| [38] | Pan, C.; Meng, Y.; Deng, Y.; Zhou, B.; Chen, J.; He, Z.; Sun, W.; Khan, R.; Fan, B. Chin. J. Chem. 2022, 40, 2040. |
| [39] | Wang, Y.; Cao, Z.; He, Q.; Huang, X.; Liu, J.; Neumann, H.; Chen, G.; Beller, M. Chem. Sci. 2023, 14, 1732. |
| [40] | Miao, M.; Zhu, L.; Zhao, H.; Song, L.; Yan, S.-S.; Liao, L.-L.; Ye, J.-H.; Lan, Y.; Yu, D.-G. Sci. China Chem. 2023, 66, 1457. |
| [41] | Masuda, Y.; Ikeshita, D.; Higashida, K.; Yoshida, M.; Ishida, N.; Murakami, M.; Sawamura, M. J. Am. Chem. Soc. 2023, 145, 19060. |
| [42] | Zhu, C.; Yue, H.; Maity, B.; Atodiresei, I.; Cavallo, L.; Rueping, M. Nat. Catal. 2019, 2, 678. |
| [43] | Long, T.; Zhu, C.; Li, L.; Shao, L.; Zhu, S.; Rueping, M.; Chu, L. Nat. Commun. 2023, 14, 55. |
| [44] | Xu, L.; Zhu, S.; Huo, L.; Chen, F.; Yu, W.; Chu, L. Org. Chem. Front. 2021, 8, 2924. |
| [45] | Li, K.; Deng, J.; Long, X.; Zhu, S. Green Chem. 2023, 25, 7253. |
| [46] | Lü, S.; Wang, Z.; Zhu, S. Nat. Commun. 2022, 13, 5001. |
| [47] | Lü, S.; Wang, Z.; Gao, X.; Chen, K.; Zhu, S. Angew. Chem. Int. Ed. 2023, 62, e202300268. |
| [48] | Yang, B.; Li, K.; Wang, Y.; Zhu, S. Sci. China Chem. 2024, 67, 936. |
| [49] | Chakrasali, P.; Kim, K.; Jung, Y.-S.; Kim, H.; Han, S. B. Org. Lett. 2018, 20, 7509. |
| [50] | Li, H.; Cheng, Z.; Tung, C.-H.; Xu, Z. ACS Catal. 2018, 8, 8237. |
| [51] | Sahoo, A. K.; Dahiya, A.; Das, B.; Behera, A.; Patel, B. K. J. Org. Chem. 2021, 86, 11968. |
| [52] | Nie, X.; Xu, T.; Hong, Y.; Zhang, H.; Mao, C.; Liao, S. Angew. Chem. Int. Ed. 2021, 60, 22035. |
| [53] | Kumar, S.; Kumar, J.; Naqvi, T.; Raheem, S.; Rizvi, M. A.; Shah, B. A. ChemPhotoChem 2022, 6, e202200110. |
| [54] | Abramov, V. A.; Topchiy, M. A.; Rasskazova, M. A.; Drokin, E. A.; Sterligov, G. K.; Shurupova, O. V.; Malysheva, A. S.; Rzhevskiy, S. A.; Beletskaya, I. P.; Asachenko, A. F. Org. Biomol. Chem. 2023, 21, 3844. |
| [55] | Ren, X.; Ke, Q.; Zhou, Y.; Jiao, J.; Li, G.; Cao, S.; Wang, X.; Gao, Q.; Wang, X. Angew. Chem. Int. Ed. 2023, 62, e202302199. |
| [56] | Xie, S.-L.; Yan, J.-Z.; Xie, M.-J.; Li, X.; Zhou, F.; Zheng, M.-Q.; Wang, X.-L.; Feng, J.; Zhang, Y.; Duan, Y.-N.; Niu, Y.-D.; Li, D.; Xia, H.-D. Green Chem. 2024, 26, 323. |
/
| 〈 |
|
〉 |