

手性噁唑硼烷催化芳基磷氧对α,β-双功能不饱和化合物的不对称膦氢化反应研究
收稿日期: 2024-05-22
网络出版日期: 2024-06-13
基金资助
国家自然科学基金(22361053); 国家自然科学基金(21961045); 云南省基础研究计划(202301AU070125); 云南省基础研究计划(202401BC070018); 云南省手性物质研究与利用重点实验室项目(202402AN360010); 云南省“兴滇英才”青年专项项目资助
Asymmetric Hydrophosphination of Diarylphosphine Oxides to α,β-Unsaturated Bifunctional Compounds Catalyzed by Chiral Oxazaborolidine
Received date: 2024-05-22
Online published: 2024-06-13
Supported by
National Natural Science Foundation of China(22361053); National Natural Science Foundation of China(21961045); Yunnan Fundamental Research Projects(202301AU070125); Yunnan Fundamental Research Projects(202401BC070018); Yunnan Key Laboratory of Chiral Functional Substance Research and Application(202402AN360010); “Yunnan Rejuvenation Talent”
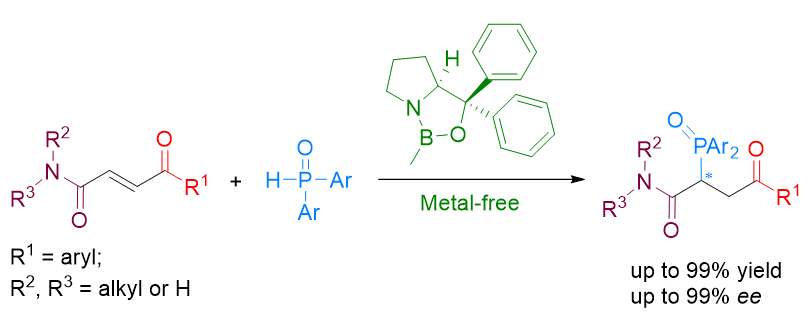
手性噁唑硼烷催化剂(CBS)作为一种经典的非金属催化剂, 被广泛地用于酮的不对称还原和不对称环加成反应. 本工作报道了一种使用CBS催化剂, 实现二芳基磷氧亲核试剂对α,β-双功能不饱和化合物的不对称Michael加成反应. 该反应的可能催化机理与早期报道的CBS催化机理不同, CBS在此反应中很有可能是作为一个路易斯酸碱对, 通过裂解亲核试剂的氢氧键, 进而通过协同加成的过程, 实现不对称膦Michael加成反应. 另外, 该反应具有底物适用性良好、反应条件温和(室温)、对空气水分不敏感、易于放大等优点, 在合成手性膦配体领域具有潜在的应用价值.
关键词: 噁唑硼烷; 芳基磷氧; α,β-不饱和化合物; 不对称膦氢化
高琦 , 陈丽蓉 , 钱金一 , 樊瑞峰 , 孙蔚青 , 郭亚飞 , 樊保敏 . 手性噁唑硼烷催化芳基磷氧对α,β-双功能不饱和化合物的不对称膦氢化反应研究[J]. 化学学报, 2024 , 82(7) : 742 -747 . DOI: 10.6023/A24050166
Oxazaborolidines (CBS) have been widely utilized as organocatalysts in enantioselective organic synthesis over the past thirty years, particularly for the asymmetric reduction of ketones and enantioselective cycloaddition reactions. The history of oxazaborolidines functioning as chiral Lewis acids has demonstrated that CBS requires activation by strong protonic acids or Lewis acids to enhance the Lewis acidity of boron, such as TfOH, Tf2NH, and AlBr3. In our previous work, we introduced a new application of CBS in the asymmetric 1,4-addition of diarylphosphine oxides to α,β-unsaturated ketones and esters. Unlike traditional CBS-catalyzed reactions, this catalytic system does not require strong protonic acids or Lewis acids to activate CBS; instead it likely functions as a Lewis pair to cleave the O—H bond and provide a chiral phosphorous intermediate. To further expand the application of this new method, we selected α,β-unsaturated bifunctional compounds bearing both a ketone and an amide group on either side of the double bond as substrates for this study. Initially, several oxazaborolidines and reaction conditions were explored for the model reaction. The results indicated that under optimal conditions (20 mol% Me-CBS at room temperature in acetonitrile for 2 h), the reaction proceeded well with high yield (99%) and high enantiomeric excess (93% ee). Subsequently, various substrates were examined under these optimal conditions yielding products with 52%~99% yields and 63%~99% ee. The general procedure involved stirring a mixture of substrates (0.24 mmol, 1.2 equiv.), diarylphosphine oxides (0.2 mmol, 1 equiv.), and CBS (20 mol% Me-CBS) in 2 mL acetonitrile for 2 h followed by purification via flash column chromatography. Finally, the potential mechanism behind how the catalyst functions as a Lewis pair to control enantioselectivity was discussed. Additionally, the chiral products have potential applications in synthesizing valuable phosphine ligands.

| [1] | Corey E. J. Angew. Chem., Int. Ed. 2002, 41, 1650. |
| [2] | (a) Hirao A.; Itsuno S.; Nakahama S.; Yamazaki N. J. Chem. Soc. Chem. Commun. 1981, 315. |
| [2] | (b) Itsuno S.; Ito K.; Hirao A.; Nakahama N. J. Org. Chem. 1984, 49, 555. |
| [3] | (a) Corey E. J.; Bakshi R. K.; Shibata S. J. Am. Chem. Soc. 1987, 109, 5551. |
| [3] | (b) Corey E. J.; Bakshi R. K.; Shibata S.; Chen C. P.; Singh V. K. J. Am. Chem. Soc. 1987, 109, 7925. |
| [4] | Liu D.; Canales E.; Corey E. J. J. Am. Chem. Soc. 2007, 129, 1498. |
| [5] | Corey E. J. Angew. Chem. Int. Ed. 2009, 48, 2100. |
| [6] | (a) Corey E. J.; Loh T. P. J. Am. Chem. Soc. 1991, 113, 8966. |
| [6] | (b) Corey E. J.; Shibata T.; Lee T. W. J. Am. Chem. Soc. 2002, 124, 3808. |
| [6] | (c) Canales E.; Corey E. J. J. Am. Chem. Soc. 2007, 129, 12686. |
| [6] | (d) Zhou G.; Corey E. J. J. Am. Chem. Soc. 2005, 127, 11958. |
| [7] | (a) Zhu R.; Liao K.; Yu J.; Zhou J. Acta Chim. Sinica 2020, 78, 193 (in Chinese). |
| [7] | (朱仁义, 廖奎, 余金生, 周剑, 化学学报, 2020, 78, 193.) |
| [7] | (b) Novas B. T.; Waterman R. ChemCatChem 2022, 14, e202200988. |
| [7] | (c) Zhang Y.; Zhu S. Acta Chim. Sinica 2023, 81, 777 (in Chinese). |
| [7] | (张艳东, 朱守非, 化学学报, 2023, 81, 777.) |
| [7] | (d) Luo C.; Yin Y.; Jiang Z. Chin. J. Org. Chem. 2023, 43, 1963 (in Chinese). |
| [7] | (罗诚, 尹艳丽, 江智勇, 有机化学, 2023, 43, 1963.) |
| [7] | (e) Zhang J.; Ni H.; Wu Q.; Yang J.; Zhang J. Chin. J. Org. Chem. 2022, 42, 3118 (in Chinese). |
| [7] | (张洁明, 倪航, 吴起, 杨俊锋, 张俊良, 有机化学, 2022, 42, 3118.) |
| [8] | (a) Feng J.; Chen X.; Shi M.; Duan W. J. Am. Chem. Soc. 2010, 132, 5562. |
| [8] | (b) Lu Z.; Zhang H.; Yang Z.; Ding N.; Meng L.; Wang J. ACS Catal. 2019, 9, 1457. |
| [8] | (c) Yue W.; Xiao J.; Zhang S.; Yin L. Angew. Chem., Int. Ed. 2020, 59, 7057. |
| [8] | (d) Pérez J. M.; Postolache R.; Castineira Reis M.; Sinnema E. G.; Vargová D.; de Vries F.; Otten E.; Ge L.; Harutyunyan S. R. J. Am. Chem. Soc. 2021, 143, 20071. |
| [8] | (e) Yu X.; Lu L.; Zhang Z.; Shi D.; Xiao W. Org. Chem. Front. 2022, 10, 133. |
| [8] | (f) Sun G.; Xiao F.; Duan W. Chin. J. Org. Chem. 2020, 40, 61 (in Chinese). |
| [8] | (孙贵救, 肖繁花, 段伟良, 有机化学, 2020, 40, 61.) |
| [8] | (g) Zhang F.; Luan Y.; Ye M. Chin. J. Org. Chem. 2021, 41, 3880 (in Chinese). |
| [8] | (张凤萍, 栾玉新, 叶萌春, 有机化学, 2021, 41, 3880.) |
| [9] | (a) Saito B.; Egami H.; Katsuki T. J. Am. Chem. Soc. 2007, 129, 1978. |
| [9] | (b) Yang F.; Zhao D.; Lan J.; Xi P.; Yang L.; Xiang S.; You J. Angew. Chem., Int. Ed. 2008, 47, 5646. |
| [9] | (c) Maiti R.; Yan J.; Yang X.; Mondal B.; Xu J.; Chai H.; Jin Z.; Chi Y. R. Angew. Chem., Int. Ed. 2021, 60, 26616. |
| [9] | (d) Hu H.; Ren X.; He J.; Zhu L.; Fang S.; Su Z.; Wang T. Sci. China Chem. 2022, 65, 2500. |
| [9] | (e) Guo F.; Chen J.; Huang Y. ACS Catal. 2021, 11, 6316. |
| [10] | Qian J.; Zhao H.; Gao Q.; Chen L.; Shi Y.; Li J.; Guo Y.; Fan B. Org. Chem. Front. 2023, 10, 5672. |
| [11] | Shi Y.; Chen L.; Gao Q.; Li J.; Guo Y.; Fan B. Org. Lett. 2023, 25, 6495. |
| [12] | Chen L.; Wang G.; Nong X.; Shao W.; Li J.; Guo Y.; Fan B. Chem. Eur. J. 2024, 30, e202401017. |
| [13] | Lu G.; Xiao L.; Que Q.; Leng T.; Li J.; Guo Y.; Fan B. J. Org. Chem. 2024, 89, 7573. |
/
| 〈 |
|
〉 |