

新型手性三齿PNN配体锰配合物催化的芳香酮类化合物的不对称氢化反应研究
收稿日期: 2024-04-08
网络出版日期: 2024-06-21
基金资助
河南省高等学校重点科研项目计划(24A150026); 河南省高等学校重点科研项目计划(23A150027); 河南省研究性教学改革研究与实践项目(GJ[2023]388111); 河南省教师教育课程改革研究项目(2024-JSJYYB-043)
Novel Chiral Tridentate PNN Ligand Manganese Complex for Enantioselective Hydrogenation of Aromatic Ketones
Received date: 2024-04-08
Online published: 2024-06-21
Supported by
Key Scientific Research Projects of Higher Education of Henan Province(24A150026); Key Scientific Research Projects of Higher Education of Henan Province(23A150027); Research and practice of teaching reform of Henan Province(GJ[2023]388111); Henan Province teacher education curriculum reform research project(2024-JSJYYB-043)
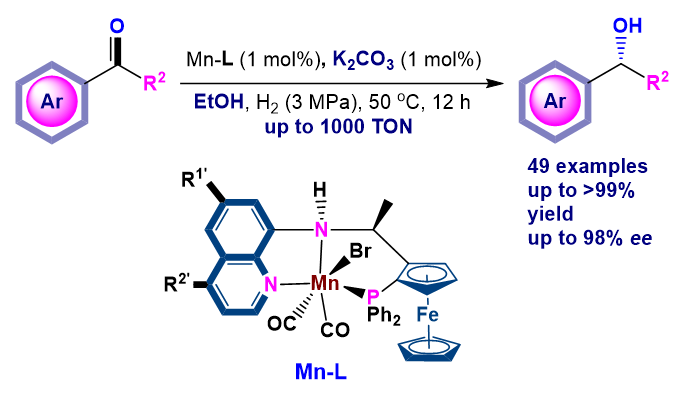
非贵金属相较于贵金属具有廉价、低毒的特点, 因此发展用于酮的不对称催化氢化的高效、低毒的非贵金属催化剂在近些年受到广泛关注, 其中手性三齿PNN和PNP配体的锰配合物在多种酮的催化不对称氢化中表现出优异的性能, 但就底物类型和催化效率而言仍有待进一步提升. 基于此, 本工作将廉价易得的8-氨基喹啉骨架引入面手性二茂铁中合成了一系列含芳胺NH的三齿PNN配体L1~L4, 并将其Mn配合物成功用于简单芳基酮、苯并环烷酮、α-氨基酮等的催化不对称氢化(达到98% ee, 1000 TON). 反应使用K2CO3作碱, EtOH作溶剂, 对(S)-phenylephrine的重要中间体β-氨基醇2-49进行克级规模制备, 证明了该催化体系具有潜在的工业应用性. 对配合物Mn-L的高分辨质谱和红外光谱的初步表征和分析, 暗示其结构中含两个CO.
江杏杏 , 武卫龙 , 任慧莹 , 张凤 , 莫文枝 , 卢志强 . 新型手性三齿PNN配体锰配合物催化的芳香酮类化合物的不对称氢化反应研究[J]. 化学学报, 2024 , 82(7) : 736 -741 . DOI: 10.6023/A24040120
Since the first-raw metals have higher metal contamination limitations in pharmaceutical compounds and cheaper. Extensive global efforts have been devoted to the development of chiral catalysts with earth abundant, inexpensive, and nontoxic transitions metals. Nevertheless, non-precious metal catalysts in ketone hydrogenation are still far from satisfactory in terms of activity, selectivity and substrate scope. Recently, much effort has been devoted to the development of powerful chiral ligands for manganese-catalyzed enantioselective ketone hydrogenation. Based on this, in this work, we synthesized a series of three-toothed PNN ligands L1~L4 by introducing 8-aminoquinoline skeleton into facial chiral ferrocene, and applied these ligands to Mn-catalyzed asymmetric hydrogenation of a broad spectrum of ketones (49 examples) with high activities (1000 TON) and high enantioselectivities (up to 98% ee) using K2CO3 and EtOH as an industrially desirable base and solvent. To further demonstrate the utility of the developed Mn catalytic system, we applied it to the catalytic asymmetric hydrogenation of α-aminoketones (1-49, 1.46 g) with gram-scale and successfully obtained β-aminoalcohols (S)-2-49 in 1.25 g, 97% isolated yield and 81% ee within 24 h at 50 ℃ under hydrogen pressure of 4 MPa. Notably, the product (S)-2-49 is a key intermediate of (S)-phenylephrine. Although our attempts to get the crystal structure of Mn-L1 failed, the data of high resolution mass spectra and infrared spectra analysis strongly supported the hypothesized structure of Mn-L1, which contains two CO molecules.

| [1] | For selected examples, see: (a) Rogawski M. A.; L?scher W. Nat. Rev. Neurosci. 2004, 5, 553. |
| [1] | (b) Creighton C. J.; Ramabadran K.; Ciccone P. E.; Liu J.; Orsini M. J.; Reitz A. B. Bioorg. Med. Chem. Lett. 2004, 14, 4083. |
| [1] | (c) Almeida L.; Soares-Da-Silva P. Neurotherapeutics 2007, 4, 88. |
| [1] | (d) Cui J. J.; Tran-Dubé M.; Shen H.; Nambu M.; Kung P.-P.; Pairish M.; Jia L.; Meng J.; Funk L.; Botrous I.; McTigue M.; Grodsky N.; Ryan K.; Padrique E.; Alton G.; Timofeevski S.; Yamazaki S.; Li Q.; Zou H.; Christensen J.; Mroczkowski B.; Bender S.; Kania R. S.; Edwards M. P. J. Med. Chem. 2011, 54, 6342. |
| [1] | (e) Yan P. C.; Zhu G. L.; Xie J. H.; Zhang X. D.; Zhou Q. L.; Li Y. Q.; Shen W. H.; Che D. Q. Org. Process Res. Dev. 2013, 17, 307. |
| [1] | (f) Qian J. Q.; Yan P. C.; Che D. Q.; Zhou Q. L.; Li Y. Q. Tetrahedron Lett. 2014, 55, 1528. |
| [1] | (g) Flick A. C.; Leverett C. A.; Ding H. X.; McInturff E.; Fink S. J.; Helal C. J.; De Forest J. C.; Morse P. D.; Mahapatra S.; O’Donnell C. J. J. Med. Chem. 2020, 63, 10652. |
| [1] | (h) He P.; Zheng H.; Liu X.; Lian X.; Lin L.; Feng X. M. Chem. Eur. J. 2014, 20, 13482. |
| [2] | For selected examples, see: (a) Noyori R.; Ohkuma T. Angew. Chem. Int. Ed. 2001, 40, 40. |
| [2] | (b) Mortreux A.; Karim A. The Handbook of Homogeneous Hydrogenation, Wiley-VCH, Weinheim, 2007. |
| [2] | (c) Yang G. Q.; Zhang W.-B. Chem. Soc. Rev. 2018, 47, 1783. |
| [2] | (d) Zhang Z. F.; Butt N. A.; Zhang W. B. Chem. Rev. 2016, 116, 14769. |
| [2] | (e) Malacea R.; Poli R.; Manoury E. Coord. Chem. Rev. 2010, 254, 729. |
| [2] | (f) Xie J. H.; Zhou Q. L. Acta Chim. Sinica 2012, 70, 1427 (in Chinese). |
| [2] | (谢建华, 周其林, 化学学报, 2012, 70, 1427.) |
| [2] | (g) Wang H.; Wen J.; Zhang X. Chem. Rev. 2021, 121, 7530. |
| [2] | (h) Alig L.; Fritz M.; Schneider S. Chem. Rev. 2019, 119, 2681. |
| [2] | (i) Zhang L.; Liu C.; Sun M.; Liang C.; Cao L.; Yao X.; Ma Y.; Cheng R.; Ye J. J. Org. Chem. 2023, 88, 2942. |
| [2] | (j) Yin C.; Jiang Y.-F.; Huang F.; Xu C.-Q.; Pan Y.; Gao S.; Chen G.-Q.; Ding X.; Bai S.-T.; Lang Q.; Li J.; Zhang X. Nat. Commun. 2023, 14, 3718. |
| [2] | (k) Bao D. H.; Wu H. L.; Liu C. L.; Xie J. H.; Zhou Q. L. Angew. Chem. Int. Ed. 2015, 54, 8791. |
| [2] | (l) Zhang F. H.; Zhang F. J.; Li M. L.; Xie J. H.; Zhou Q. L. Nat. Catal. 2020, 3, 621. |
| [2] | (m) Wu W. L.; Zhao N.; Liu Y.; Du S.; Wang X.; Mo W.; Yan X.; Xu C.; Zhou Y.; Ji B. Org. Lett. 2023, 25, 8845. |
| [3] | For a review, see: (a) Zhang Z.; Butt N. A.; Zhou M.; Liu D.; Zhang W. Chin. J. Chem. 2018, 36, 443. |
| [3] | (b) Filonenko G. A.; van Putten R.; Hensen E. J. M.; Pidko E. A. Chem. Soc. Rev. 2018, 47, 1459. |
| [4] | (a) Shimizu H.; Igarashi D.; Kuriyama W.; Yusa Y.; Sayo N.; Saito T. Org. Lett. 2007, 9, 1655. |
| [4] | (b) Junge K.; Wendt B.; Addis D.; Zhou S.; Das S.; Fleischer S.; Beller M. Chem. Eur. J. 2011, 17, 101. |
| [4] | (c) Krabbe S. W.; Hatcher M. A.; Bowman R. K.; Mitchell M. B.; McClure M. S.; Johnson J. S. Org. Lett. 2013, 15, 4560. |
| [4] | (d) Zatolochnaya O. V.; Rodríguez S.; Zhang Y.; Lao K. S.; Tcyrulnikov S.; Li G.; Wang X.-J.; Qu B.; Biswas S.; Mangunuru H. P. R.; Rivalti D.; Sieber J. D.; Desrosiers J.-N.; Leung J. C.; Grinberg N.; Lee H.; Haddad N.; Yee N. K.; Song J. J.; Kozlowski M. C.; Senanayakea C. H. Chem. Sci. 2018, 9, 4505. |
| [5] | (a) Hamada Y.; Koseki Y.; Fujii T.; Maeda T.; Hibino T.; Makino K. Chem. Commun. 2008, 6206. |
| [5] | (b) Hibino T.; Makino K.; Sugiyama T.; Hamada Y. ChemCatChem 2009, 1, 237. |
| [5] | (c) Cai X. H.; Chen J. Z.; Zhang W. B. Acta Chim. Sinica 2023, 81, 646 (in Chinese). |
| [5] | (蔡新红, 陈建中, 张万斌, 化学学报, 2023, 81, 646.) |
| [5] | (d) Ding Y. X.; Zhou Y. G. Chin. J. Org. Chem. 2022, 42, 2994 (in Chinese). |
| [5] | (丁艺璇, 周永贵, 有机化学, 2022, 42, 2994.) |
| [6] | (a) Berkessel A.; Reichau S.; von der H?h A.; Leconte N.; Neud?rfl J.-M. Organometallics 2011, 30, 3880. |
| [6] | (b) Gajewski P.; Renom-Carrasco M.; Facchini S. V.; Pignataro L.; Lefort L.; de Vries J. G.; Ferraccioli R.; Forni A.; Piarulli U.; Gennari C. Eur. J. Org. Chem. 2015, 1887. |
| [6] | (c) Hodgkinson R.; Del Grosso A.; Clarkson G. J.; Wills M. Dalton Trans. 2016, 3992. |
| [7] | (a) Zhang D.; Zhu E.-Z.; Lin Z.-W.; Li Y.-Y.; Gao J.-X. Asian J. Org. Chem. 2016, 5, 1323. |
| [7] | (b) Friedfeld M. R.; Shelvin M.; Hoyt J. M.; Krska S. W.; Tudge M. T.; Chirik P. J. Science 2013, 342, 1076. |
| [7] | (c) Friedfeld M. R.; Margulieux G. W.; Schaefer B.; Chirik P. J. J. Am. Chem. Soc. 2014, 136, 13178. |
| [7] | (d) Chirik P. J. Acc. Chem. Res. 2015, 48, 1687. |
| [7] | (e) Friedfeld M. R.; Zhong H.; Ruck R. T.; Shelvin M.; Chirik P. J. Science 2018, 360, 888. |
| [7] | (f) Monfette S.; Turner Z. R.; Semproni S. P.; Chirik P. J. J. Am. Chem. Soc. 2012, 134, 4561. |
| [7] | (g) Friedfeld M. R.; Shevlin M.; Margulieux G. W.; Campeau L. C.; Chirik P. J. J. Am. Chem. Soc. 2016, 138, 3314. |
| [7] | (h) Du T. Wang B.; Wang C.; Xiao J.; Tang W. Chinese Chem. Lett. 2021, 32, 1241. |
| [7] | (i) Elangovan S.; Topf C.; Fischer S.; Jiao H.; Spannenberg A.; Baumann W.; Ludwig R.; Junge K.; Beller M. J. Am. Chem. Soc. 2016, 138, 8809. |
| [8] | (a) Wang Y.; Zhu L.; Shao Z.; Li G.; Lan Y.; Liu Q. J. Am. Chem. Soc. 2019, 141, 17337. |
| [8] | (b) Kallmeier F.; Kempe R. Angew. Chem. Int. Ed. 2018, 57, 46. |
| [8] | (c) Garbe M.; Junge K.; Beller M. Eur. J. Org. Chem. 2017, 4344. |
| [8] | (d) Maji B.; Barman M. K. Synthesis 2017, 49, 3377. |
| [8] | (e) Gulyaeva E. S.; Osipova E. S.; Buhaibeh R.; Canac Y.; Sortais J. B.; Valyaev D. A. Coord. Chem. Rev. 2022, 458, 21442. |
| [8] | (f) Das K.; Waiba S.; Jana A.; Maji B. Chem. Soc. Rev. 2022, 51, 4386. |
| [8] | (g) Wang Y.; Wang M.; Li Y.; Liu Q. Chem 2021, 7, 1180. |
| [9] | Widegren M. B.; Harkness G. J.; Slawin A. M. Z.; Cordes D. B.; Clarke M. L. Angew. Chem. Int. Ed. 2017, 56, 5825. |
| [10] | (a) Yang J.; Yao L.; Wang Z.; Zuo Z.; Liu S.; Gao P.; Han M.; Liu Q.; Solan G. A.;Sun. W. -H. J. Catal. 2023, 418, 40. |
| [10] | (b) Oates C. L.; Goodfellow A. S.; Bühl M.; Clarke M. L. Green Chem. 2023, 25, 3864. |
| [10] | (c) He J.; Mao W.; Lin J.; Wu Y.; Chen L.; Yang P.; Song D.; Zhu P.; Zhong W.; Ling F. Org. Chem. Front. 2023, 10, 3321. |
| [10] | (d) Wang Z.; Zhao X.; Huang A.; Yang Z.; Cheng Y.; Chen J.; Ling F.; Zhong W. Tetrahedron Lett. 2021, 82, 153389. |
| [10] | (e) Widegren M. B.; Clarke M. L. Catal. Sci. Technol. 2019, 9, 6047. |
| [10] | (f) Passera A.; Mezzetti A. Adv. Synth. Catal. 2019, 361, 4691. |
| [10] | (g) Zhang L.; Wang Z.; Han Z.; Ding K. Angew. Chem. Int. Ed. 2020, 59, 15565. |
| [10] | (h) Seo C. S. G.; Tsui B. T. H.; Gradiski M. V.; Smith S. A. M.; Morris R. H. Catal. Sci. Technol. 2021, 1, 3153. |
| [11] | Garbe M.; Junge K.; Walker S.; Wei Z.; Jiao H.; Spannenberg A.; Bachmann S.; Scalone M.; Beller M. Angew. Chem. Int. Ed. 2017, 56, 11237. |
| [12] | Zhang L.; Tang Y.; Han Z.; Ding K. Angew. Chem. Int. Ed. 2019, 58, 4973. |
| [13] | (a) Ling F.; Hou H.; Chen J.; Nian S.; Yi X.; Wang Z.; Song D.; Zhong W. Org. Lett. 2019, 21, 3937. |
| [13] | (b) Ling F.; Chen J.; Nian S.; Hou H.; Yi X.; Wu F.; Xu M.; Zhong W. Synlett 2020, 31, 285. |
| [14] | Zeng L.; Yang H.; Zhao M.; Wen J.; Tucker J. H. R.; Zhang X. ACS Catal. 2020, 10, 13794. |
| [15] | (a) Yuan M.-L.; Xie J. H.; Yang X.-H.; Zhou Q. L. Synthesis 2014, 46, 2910. |
| [15] | (b) Hu Y.; Wu W.; Dong X.-Q.; Zhang X. Org. Chem. Front. 2017, 4, 1499. |
/
| 〈 |
|
〉 |