

Zn, C引入量和煅烧温度对ZnO/C/CeO2光催化还原Cu2+效率的影响
收稿日期: 2024-04-25
网络出版日期: 2024-07-22
基金资助
国家自然科学基金(21876158); 浙江省金华市重点科技项目(2022-1-077); 浙江省新苗人才计划(2023R404052)
Effect of Zn, C Introduction Amount and Calcination Temperature on the Photocatalytic Reduction of Cu2+over ZnO/C/CeO2
Received date: 2024-04-25
Online published: 2024-07-22
Supported by
National Natural Science Foundation of China(21876158); Key Science and Technology Projects in Jinhua City of Zhejiang Province(2022-1-077); Zhejiang Province New Talent Plan(2023R404052)
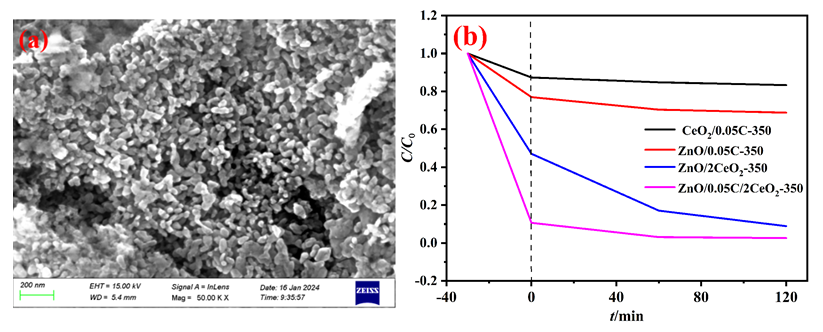
以Ce(NO3)3•6H2O和Zn(NO3)2•6H2O为金属源, 酸性红14 (AR14)为碳源, 通过共沉积法制备了Zn(OH)2/AR14/Ce(OH)4前驱体, 在惰性气体中煅烧前驱体获得ZnO/C/CeO2材料. 采用X射线衍射、扫描电子显微镜、红外光谱、光致发光、X射线光电子能谱等技术对合成材料进行了表征, 结果表明, 所得材料中Zn、Ce、C分布均匀, 在CeO2中引入Zn和C有利于氧空位和碳键形成, 促进光电子和空穴有效分离, 从而提高产物光催化活性. 通过Cu2+还原考察了主要合成参数对ZnO/C/CeO2光催化活性的影响, 结果表明, 当反应原料中Zn(NO3)2•6H2O加入量为0.5 g, AR14浓度为0.05 mmol/L, 煅烧温度为350 ℃时, 所制备的ZnO/0.05C/2CeO2对Cu2+的光催化还原效率最好, 达到95.34%.
关键词: ZnO/C/CeO2; Cu2+; 光催化还原; 氧空位; 电子-空穴对
张帆帆 , 蔡元韬 , 陶剑波 , 常国菊 , 郭欣辰 , 郝仕油 . Zn, C引入量和煅烧温度对ZnO/C/CeO2光催化还原Cu2+效率的影响[J]. 化学学报, 2024 , 82(8) : 871 -878 . DOI: 10.6023/A24040144
In this study, the Zn(OH)2/AR14/Ce(OH)4 precursor was prepared by a co-deposition method using Ce(NO3)3•6H2O as the cerium source, Zn(NO3)2•6H2O as the zinc source, and acidic red 14 (AR14) as the carbon source. Then, ZnO/C/CeO2 materials were obtained by calcination of the precursor in a tube furnace under N2 atmosphere at different temperatures. The composite was characterized by X-ray diffraction, scanning electron microscope, Fourier transform infrared spectroscopy, photoluminescence and X-ray photoelectron spectroscopy. The results showed that the ternary composites were prepared successfully, and that the carbon existed in an amorphous form. Due to the thermal decomposition of the precursor at high temperatures, the synthesized products exhibit porous property. Due to the uniform distribution of reactants in the reaction system, the distribution of Zn, Ce and C in the obtained material was uniform. It can be seen that the introduction of Zn and the formation of Ce3+ in CeO2 are conducive to the formation of oxygen vacancies, generating intermediate energy levels and thus broadening the light absorption range. Furthermore, oxygen vacancies can easily capture photoelectrons and thus accelerate their separation from holes in the value band, which can improve the photocatalytic efficiency of the resulted materials. The introduction of C into CeO2 can produce a certain amount of carbon bonds, promote the effective separation of photoelectrons and holes, and improve the photocatalytic activity of the product. The effects of the amount of introduced Zn and C and the calcination temperature on the photocatalytic activity of ZnO/C/CeO2 were investigated by the photocatalytic reduction of Cu2+. The results showed that when the amount of Zn(NO3)2•6H2O in the reaction raw material was 0.5 g, the concentration of AR14 was 0.05 mmol/L, and the calcination temperature was 350 ℃ for the precursor, the photocatalytic reduction efficiency of Cu2+ over ZnO/C/CeO2 is the highest, reaching 95.34%. The main reasons for the best photocatalytic performance are the highest oxygen vacancy concentration and maximum number of carbon bonds in ZnO/0.05C/2CeO2-350, resulting in the highest separation efficiency of photogenerated electrons and holes.

Key words: ZnO/C/CeO2; Cu2+; photocatalytic reduction; oxygen vacancy; electron-hole pair
| [1] | Fu, F.; Wang, Q. J. Environ. Manage. 2011, 92, 407. |
| [2] | Aziz, H. A.; Adlan, M. N.; Ariffin, K. S. Biores. Technol. 2008, 99, 1578. |
| [3] | Huang, Z.; Lu, L.; Cai, Z. X.; Ren, Z. J. J. Hazard. Mater. 2016, 302, 323. |
| [4] | Ihsanullah.; Abbas, A.; Al-Amer, A. M.; Laoui, T.; Al-Marri, M. J.; Nasser, M. S.; Khraisheh, M.; Atieh, M. A. Sep. Purif. Technol. 2016, 157, 141. |
| [5] | Dong, C. C.; Lu, J.; Qiu, B. C.; Shen, B.; Xing, M. Y.; Zhang, J. L. Appl. Catal., B 2018, 222, 146. |
| [6] | Xu, Z.; Shan, C.; Xie, B. H.; Liu, Y.; Pan, B. C. Appl. Catal., B 2017, 200, 439. |
| [7] | Joseph, H. M.; Poornima, N. Mater. Today 2019, 9, 7. |
| [8] | Zhong, W.; Xia, Y. F.; Zhai, H. L.; Gao, Y.; Li, S. H.; Lv, C. X. Chinese J. Inorg. Chem. 2020, 36, 40 (in Chinese). |
| [8] | (钟伟, 夏颖帆, 翟杭玲, 高越, 李世慧, 吕春欣, 无机化学学报, 2020, 36, 40.) |
| [9] | Umar, A.; Kumar, R.; Akhtar, M. S.; Kumar, G.; Kim, S. H. J. Colloid Interf. Sci. 2015, 454, 61. |
| [10] | Li, C. Q.; Luo, L. T.; Xiong, G. W. Acta Chim. Sinica 2010, 68, 1023 (in Chinese). |
| [10] | (李长全, 罗来涛, 熊光伟, 化学学报, 2010, 68, 1023.) |
| [11] | Pathak, V.; Lad, P.; Thakkar, A. B.; Thakor, P.; Deshpande, M. P.; Pandya, S. Inorg. Chem. Commun. 2024, 159, 111738. |
| [12] | Sun, X. C.; Yuan, K.; Hua, W. D.; Gao, Z. R.; Zhang, Q.; Yuan, C. Y.; Liu, H. C.; Zhang, Y. W. ACS Catal. 2022, 12, 11942. |
| [13] | Celebi, N.; Salimi, K. J. Colloid Interf. Sci. 2022, 605, 23. |
| [14] | Yu, L. X.; Yang, L. Y.; Chen, H. S.; Tao, J. B.; Hao, S. Y. J. Rare Earths 2021, 39, 238 (in Chinese). |
| [14] | (余丽霞, 杨丽媛, 陈寒松, 陶剑波, 郝仕油, 中国稀土学报, 2021, 39, 238.) |
| [15] | Wang, H.; Shang, J.; Xiao, Z. L.; Aprea, P.; Hao, S. Y. Dyes Pigm. 2020, 182, 108669. |
| [16] | Sun, Q. P.; He, Z. W.; Tan, H. Y.; Aprea, P.; Hao, S. Y. Ceram. Int. 2022, 48, 26846. |
| [17] | Ahmad, N.; Umar, A.; Kumar, R.; Alam, M. Ceram. Int. 2016, 42, 11562. |
| [18] | Manikanika, L.; Chopra, L.; Kumar, R. Inorg. Chem. Commun. 2024, 160, 111896. |
| [19] | Chen, Y.; Zhang, L.; Liu, Z.; Qiu, K. H. Chem. Eng. Technol. 2017, 7, 11 (in Chinese). |
| [19] | (陈越, 张力, 刘铸, 邱克辉, 化学工程与技术, 2017, 7, 11.) |
| [20] | Imtiaz, A.; Farrukh, M. A.; Latif, H. J. Mol. Struct. 2024, 1300, 137174. |
| [21] | Xu, D. Y.; Cheng, F.; Lu, Q. Z.; Dai, P. Ind. Eng. Chem. Res. 2014, 53, 2625. |
| [22] | Sabzehmeidani, M. M.; Karimi, H.; Ghaedi, M. New J. Chem. 2020, 44, 5033. |
| [23] | Yue, L.; Zhang, X. M. J. Alloys Compd. 2009, 475, 702. |
| [24] | Wang, X. Q.; Rodriguez, J. A.; Hanson, J. C.; Gamarra, D.; Martinez-Arias, A.; Fernandez-Garcia, M. J. Phys. Chem. B 2005, 109, 19595. |
| [25] | Albrecht, P. M.; Jiang, D. E.; Mullins, D. R. J. Phys. Chem. C 2014, 118, 9042. |
| [26] | Zhang, P.; Zhang, H. W.; Wang, S. H.; Lei, X. Q.; Yang, J. T.; Li, Z. M.; Zhu, H. B.; Bao, X. J.; Yuan, P. J. Mater. Sci. 2020, 55, 12876. |
| [27] | Jiang, H. Y.; Liu, G. G.; Li, M.; Liu, J. J.; Sun, W. B.; Ye, J. H.; Lin, J. Appl. Catal., B 2015, 163, 267. |
| [28] | Chen, S. B.; Li, X.; Zhou, W. Y.; Zhang, S. S.; Fang, Y. P. Appl. Surf. Sci. 2018, 466, 254. |
| [29] | Lei, W. J.; Wang, H.; Zhang, X. J.; Yang, Z. M.; Kong, C. C. Ceram. Int. 2022, 48, 1757. |
| [30] | Zhang, X.; Matras-Postolek, K.; Yang, P.; Jiang, S. P. J. Colloid Interf. Sci. 2023, 636, 646. |
| [31] | Xue, Y. F.; Tian, D.; Zhang, D. X.; Zeng, C. H.; Fu, Y. C.; Li, K. Z.; Wang, H.; Tian, Y. F. Comput. Mater. Sci. 2019, 158, 197. |
| [32] | Ma, D. D.; Sun, D. K.; Zou, Y. J.; Mao, S. M.; Lv, Y. X.; Wang, Y.; Li, J.; Shi, J. W. J. Colloid Interf. Sci. 2019, 549, 179. |
| [33] | Chen, M. Y.; Zu, X. T.; Xiang, X.; Zhang, H. L. Physica B 2006, 389, 263. |
| [34] | Laguna, O. H.; Centeno, M. A.; Boutonnet, M.; Odriozola, J. A. Appl. Catal., B 2011, 106, 621. |
| [35] | Xu, J.; Li, M.; Qiu, J. H.; Zhang, X. F.; Feng, Y.; Yao, J. F. Chem. Eng. J. 2020, 383, 123135. |
| [36] | Zangeneh, H.; Farhadian, M.; Zinatizadeh, A. A. J. Environ. Chem. Eng. 2020, 8, 103639. |
| [37] | Vignesh, S.; Suganthi, S.; Sundar, J. K.; Raj, V. Appl. Surf. Sci. 2019, 488, 763. |
| [38] | Zheng, X. G.; Chen, Q.; Lv, S. H.; Fu, X. J.; Wen, J.; Liu, X. H. Nanomaterials 2019, 9, 1643. |
| [39] | Wen, W. J.; Lou, Z. C.; Chen, Y. J.; Chen, D. S.; Tian, S. H.; Xiong, Y. J. Chem. Technol. Biotechnol. 2019, 94, 1576. |
| [40] | Tan, H. Y.; Xiao, Z. L.; Jiang, S. J.; Sun, Q. P.; Hao, S. Y. Acta Scien. Circum. 2023, 43, 77 (in Chinese). |
| [40] | (谭贺云, 肖忠连, 蒋森杰, 孙巧萍, 郝仕油, 环境科学学报, 2023, 43, 77.) |
/
| 〈 |
|
〉 |