

氮修饰Ni2P-Nx/SiO2催化剂设计合成及间甲酚加氢脱氧性能研究
Preparation of N Modified Ni2P-Nx/SiO2 Catalysts and Hydrodeoxygenation Performance of m-Cresol
Received date: 2024-07-15
Online published: 2024-09-06
Supported by
National Natural Science Foundation of China(22278068)
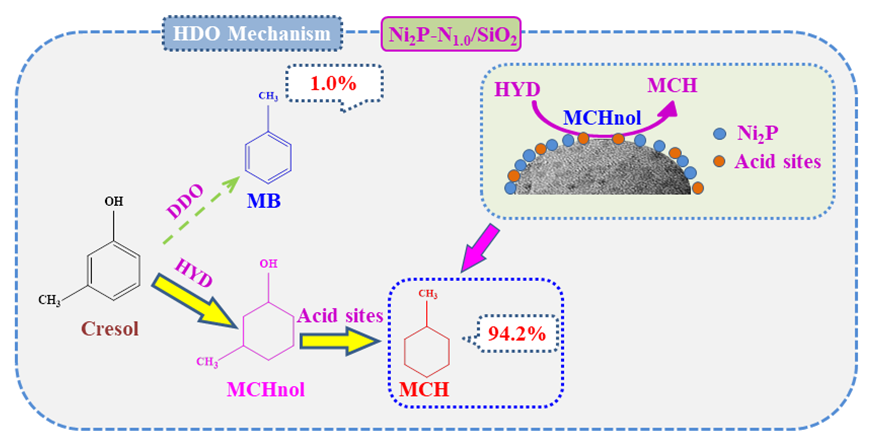
采用浸渍法制备了一系列N修饰的Ni2P-Nx/SiO2催化剂, 通过X射线衍射(XRD)、N2物理吸脱附(BET)、H2-程序升温还原(H2-TPR)、NH3-程序升温脱附(NH3-TPD)、吡啶红外(Py-FTIR)、透射电子显微镜(TEM)、X射线光电子能谱(XPS)等表征手段对催化剂的结构进行分析, 系统地研究了Ni/N物质的量比对催化剂理化性质的影响. 以间甲酚为模型化合物, 研究了Ni/N物质的量比、反应温度、反应时间及反应压力对催化剂加氢脱氧(HDO)性能的影响. 结果表明, 适量N修饰可以促进更小活性颗粒的形成, 提高活性组分的分散性, 进而提高催化剂的催化活性. Ni/N比为1时制备的Ni2P-N1.0/SiO2催化剂具有最优异的HDO活性, 在250 ℃、3 MPa、1 h条件下, 间甲酚转化率为93.2%, 目标产物甲基环己烷(MCH)的选择性达到94.2%. Ni2P-Nx/SiO2催化剂上间甲酚HDO主要以加氢-脱氧(HYD)路径为主.
关键词: N修饰; Ni2P-Nx/SiO2催化剂; 间甲酚; 加氢脱氧; 甲基环己烷
王帅 , 宋华 . 氮修饰Ni2P-Nx/SiO2催化剂设计合成及间甲酚加氢脱氧性能研究[J]. 化学学报, 2024 , 82(9) : 979 -986 . DOI: 10.6023/A24070217
The gradual depletion of fossil fuels and increasing environmental pollution problems inspire researchers to seek renewable and clean energy. Lignocellulosic biomass is deemed to be a sustainable and promising resource to produce chemicals and fuels due to its abundant existence in nature. Hydrodeoxygenation (HDO) of phenolic compounds in bio-fuel is a promising technology to convert biomass materials to value-added chemicals and fuels. However, the development of highly efficient catalysts remains a great challenge. In this work, a series of N modified Ni2P-Nx/SiO2 catalysts were prepared by impregnation method. The structure of the catalysts was characterized by X-ray diffraction (XRD), N2 physical adsorption desorption (BET), H2 temperature programmed reduction (H2-TPR), NH3 temperature programmed desorption (NH3-TPD), pyridine infrared (Py-FTIR), transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS), and the influence of Ni/N molar ratio on the physicochemical properties of the catalysts was systematically investigated. Using m-cresol as a model compound, the effects of Ni/N molar ratio, reaction temperature, reaction time, and reaction pressure on the HDO performance were studied. It was found that the appropriate introduction of N element can promote the formation of smaller active particles, improve the dispersion of active components, thus enhancing the catalytic activity. The Ni2P-N1.0/SiO2 catalyst with a Ni/N ratio of 1.0 exhibited the best HDO activity. Under the conditions of 250 ℃, 3 MPa, and 1 h, m-cresol conversion reached 93.2%, and the selectivity of the target product methylcyclohexane (MCH) reached 94.2%. Based on the product distribution, it can be inferred HDO of m-cresol over Ni2P-Nx/SiO2 catalyst proceeded mainly via hydrogenation-deoxygenation (HYD) pathway. Furthermore, this series of catalysts also displayed excellent recyclability, m-cresol conversion, as well as the selectivity towards MCH did not decrease obviously even after 5 successive cycles. We believe that this strategy can provide a simple and repeatable route to synthesize high-performance HDO catalysts.

Key words: N modified; Ni2P-Nx/SiO2 catalyst; m-cresol; hydrodeoxygenation; methylcyclohexane
| [1] | Chen S.; Miao C.; Xie H.; Jiao Z.; Zhang X.; Zhou G. Biomass Bioenergy 2024, 180, 107002. |
| [2] | Wang S.; Jiang N.; Zhu T.; Zhang Q.; Zhang C.; Wang H.; Chen Y.; Li F.; Song H. Catal. Sci. Technol. 2022, 12, 1586. |
| [3] | Zhang Q.; Wang S.; Jiang, N ; Jiang, B.; Liu Y.; Chen Y.; Li F.; Song H. J. Catal. 2024, 432, 115338. |
| [4] | Liu L.; Zhang J.; Wang L.; Xiao F. Acta Chim. Sinica 2023, 81, 533 (in Chinese). |
| [4] | (刘露杰, 张建, 王亮, 肖丰收, 化学学报, 2023, 81, 533.) |
| [5] | Li Z.; Ren X.; Wang Y.; Li Z.; Ma S.; Qiu Z. Journal of Molecular Catalysis 2022, 36, 42 (in Chinese). |
| [5] | (李志勤, 任枭雄, 王元哲, 李宗轩, 马少博, 邱泽刚, 分子催化, 2022, 36, 42.) |
| [6] | Ochoa E.; Torres D.; Pinilla J. L.; Suelves I. J. Environ. Chem. Eng. 2021, 9, 105146. |
| [7] | LiBretto N.; Tacey S.; Zubair M.; Bui T.; Unocic K.; Baddour F.; Griffin M.; Schaidle J.; Farberow C.; Ruddy D.; Bedford N.; Habas S. J. Mater. Chem. A 2023, 11, 16788. |
| [8] | Pitakjakpipop P.; Song C. Energy Fuels 2023, 37, 8311. |
| [9] | Jiang B.; Zhang Y. ; Wang H.; Liu K.; Jiang N.; Li J.; Song H. Chem. Eng. J. 2024, 495, 153118. |
| [10] | Gou X.; Jiang B.; Zhu T.; Zhang Q.; Wang S.; Li F.; Wang H.; Song H. Fuel 2024, 375, 32580. |
| [11] | Zhu T.; Liu K.; Wang H.; Wang J.; Li F.; Wang C.; Song H. Fuel 2023, 331, 125663. |
| [12] | Xu B.; Wei X.; Sun J.; Liu J.; Ma L. Acta Chim. Sinica 2023, 81, 239 (in Chinese). |
| [12] | (徐斌, 韦秀芝, 孙江敏, 刘建国, 马隆龙, 化学学报, 2023, 81, 239.) |
| [13] | Wang S.; Li F.; Liu Y.; Zhang Q.; Song H. New J. Chem. 2022, 46, 16941. |
| [14] | Ning X.; Sun Y.; Fu H.; Qu X.; Xu Z.; Zheng S. Chemosphere 2020, 241, 124978. |
| [15] | Zhang Z.; Tang M.; Chen J. Appl. Surf. Sci. 2016, 360, 353. |
| [16] | Zhao H.; Liu C.; Zheng Y.; Li S.; Gao Y.; Ma Q.; Wang F.; Dong Z. ACS Catal. 2024, 14, 8619. |
| [17] | Feitosa L. F.; Berhault G.; Laurenti D.; Teixeira da Silva V. Ind. Eng. Chem. Res. 2019, 58, 16164. |
| [18] | Zhou M.; Ye J.; Liu P.; Xu J.; Jiang J. ACS Sustainable Chem. Eng. 2017, 5, 8824. |
| [19] | Zhang Q.; Wang H.; Wang S.; Wang Y.; Zhang M.; Song H. Acta Chim. Sinica 2024, 82, 287 (in Chinese). |
| [19] | (张强, 王欢, 王帅, 王园园, 张梅, 宋华, 化学学报, 2024, 82, 287.) |
| [20] | Wang W.; Wang X.; Wang Y.; Jiang B.; Song H. React. Chem. Eng. 2022, 7, 978. |
| [21] | Wu C.; Kopold P.; Aken P. A.; Maier J.; Yu Y. Adv. Mater. 2017, 29, 1604015. |
| [22] | Wang M.; Lin M.; Li J.; Huang L.; Zhuang Z.; Lin C.; Zhou L.; Mai L. Chem. Commun. 2017, 53, 8372. |
| [23] | Jampa S.; Puente-Urbina A.; Ma Z. Q.; Wongkasemjit S.; Luterbacher J. S.; van Bokhoven J. A. ACS Sustainable Chem. Eng. 2019, 7, 4058. |
| [24] | Antar M.; Lyu D. M.; Nazari M.; Shah A.; Zhou X. M.; Smith D. L. Renew. Sust. Energy Rev. 2021, 139, 110691. |
| [25] | Kabir G.; Hameed B. H. Renew. Sust. Energy Rev. 2017, 70, 945. |
/
| 〈 |
|
〉 |