

使用机械化学生成的钙基重格氏试剂(R-CaX)对有机卤化物进行直接硼化
收稿日期: 2024-09-11
网络出版日期: 2024-10-17
基金资助
中国博士后科学基金(2021M692713); 江苏省自然科学基金(BK20210789)
Direct Borylation of Organohalides Using Mechanochemically Generated Calcium-Based Heavy Grignard Reagents (R-CaX)
Received date: 2024-09-11
Online published: 2024-10-17
Supported by
China Postdoctoral Science Foundation(2021M692713); Natural Science Foundation of Jiangsu Province(BK20210789)
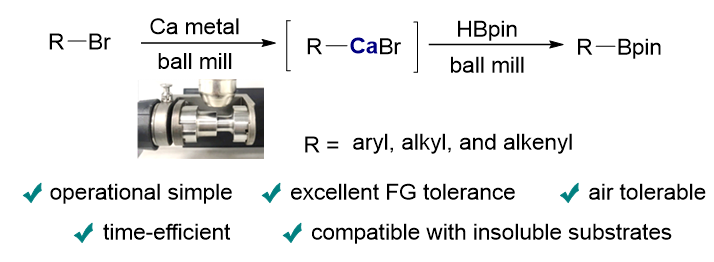
钙基重格氏试剂(R-CaX)的研究由于其合成难度大, 至今应用较少. 钙基重格氏试剂相较于镁基格氏试剂(R-MgX)而言, 碳金属键具备更强的极性和离子化特性, 因此可以在某些转化中表现出独特的反应活性. 传统合成钙基重格氏试剂需对金属钙进行预先活化, 例如使用有毒的液氨或者高活性的金属锂, 这些苛刻的反应条件严重阻碍了其在实验室和工业生产中的应用. 本研究利用球磨机产生的持续的机械能破坏金属钙表面的惰性氧化层, 实现钙的原位活化, 随后直接插入卤代烃的碳卤键, 原位生成相应的钙基重格氏试剂. 随后, R-CaX作为碳亲核试剂与频哪醇硼烷(HBpin)反应, 成功合成了有机硼化合物, 并具有良好的底物兼容性; 特别是在使用难溶溴代芳烃作为底物时, 该机械化学方法与传统溶液反应体系相比, 展现出独特的优势.
刘雨涵 , 高盼 . 使用机械化学生成的钙基重格氏试剂(R-CaX)对有机卤化物进行直接硼化[J]. 化学学报, 2024 , 82(11) : 1114 -1119 . DOI: 10.6023/A24090273
The study of calcium-based Grignard reagents (R-CaX) has historically been limited due to the challenges in their synthesis. However, calcium’s biocompatibility and distinct chemical properties make it a highly promising candidate for organic synthesis applications. Unlike their magnesium-based counterparts (R-MgX), calcium-based reagents exhibit stronger polarity and a higher degree of ionic character in the carbon-metal bond. This increased ionic nature can result in unique reactivity, offering advantages in specific transformations where magnesium-based reagents may be less effective. Traditional methods for synthesizing calcium-based Grignard reagents are cumbersome, requiring the pre-activation of metallic calcium through processes involving toxic materials such as liquid ammonia or highly reactive lithium. These harsh conditions not only present safety concerns but also limit the reagents' practicality, particularly in industrial applications where scalability and safety are crucial. In recent years, mechanochemistry has emerged as a sustainable alternative to conventional methods, providing opportunities to improve reaction efficiency and reduce the use of solvents. The mechanical energy generated by ball milling has been shown to disrupt the inert surface layer of metallic calcium, allowing for in situ activation. This enables the calcium to react with organohalides, forming calcium-based Grignard reagents without the need for toxic reagents or complex pre-activation steps. Once formed, these reagents can act as carbon nucleophiles, reacting with pinacolborane (HBpin) to form organoboron compounds, which are valuable intermediates in organic synthesis. This study demonstrates that by utilizing the continuous mechanical energy of ball milling, a variety of boronated products can be synthesized efficiently and with high yields. The method also shows broad substrate compatibility, particularly excelling in reactions involving poorly soluble aryl bromides, where traditional solution-based systems often fail. In conclusion, this work marks the first successful application of calcium-based Grignard reagents in borylation reactions via mechanochemical methods, offering a novel and efficient approach that addresses the limitations of conventional techniques.

| [1] | Hanusa, T. P. Chem. Rev. 1993, 93, 1023. |
| [2] | The Chemistry of Organo-magnesium Compounds, Eds.: Rappoport, Z.; Marek, I., Wiley-VCH, Weinheim, Germany, 2008. |
| [3] | (a) Banno, T.; Hayakawa, Y.; Umeno, M. J. Organomet. Chem. 2002, 653, 288. |
| [3] | (b) Peltzer, R. M.; Gauss, J.; Eisenstein, O.; Cascella, M. J. Am. Chem. Soc. 2020, 142, 2984. |
| [4] | Harder, S. Chem. Rev. 2010, 110, 3852. |
| [5] | Holleman, A. F.; Wiberg, E.; Wiberg, N. Inorganic Chemistry, De Gruyter, Berlin, 2001. |
| [6] | (a) Wu, T.-C.; Xiong, H.; Rieke, R. D. J. Org. Chem 1990, 55, 5045. |
| [6] | (b) Mochida, K.; Ogawa, H. J. Organomet. Chem. 1983, 243, 131. |
| [6] | (c) Li, H.; Wang, X.-Y.; Wei, B.; Xu, L.; Zhang, W.-X.; Pei, J.; Xi, Z. Nat. Commun. 2014, 5, 4508. |
| [6] | (d) Westerhausen, M.; Koch, A.; G?rls, H.; Krieck, S. Chem.-Eur. J. 2017, 23, 1456. |
| [7] | Beckmann, E. Ber. Dtsch. Chem. Ges 1905, 38, 904. |
| [8] | (a) Gilman, H.; Schulze, F. J. Am. Chem. Soc 1926, 48, 2463. |
| [8] | (b) Bryce-Smith, D.; Skinner, A. C. J. Chem. Soc 1963, 577. |
| [8] | (c) Kawabata, N.; Matsumura, A.; Yamashita, S. Tetrahedron 1973, 29, 1069. |
| [8] | (d) Kawabata, N.; Matsumura, A.; Yamashita, S. J. Org. Chem 1973, 38, 4268. |
| [9] | (a) Langer, J.; Krieck, S.; Fischer, R.; G?rls, H.; Walther, D.; Westerhausen, M. Organometallics 2009, 28, 5814. |
| [9] | (b) Maercker, A. Angew. Chem. Int. Ed. 1987, 26, 972. |
| [10] | Westerhausen, M.; Langer, J.; Krieck, S.; Fischer, R.; G?rls, H.; K?hler, M. Top. Organomet. Chem 2013, 45, 29. |
| [11] | D'Alterio, M. C.; Casals-Crua?as, è.; Tzouras, N. V.; Talarico, G.; Nolan, S. P.; Poater, A. Chem. Eur. J 2021, 27, 13481. |
| [12] | Baker, R. H.; Schlesinger, A. H. J. Am. Chem. Soc 1945, 67, 1499. |
| [13] | Chen, J.-Q.; Li, J.-H.; Dong, Z.-B. Adv. Synth. Catal 2020, 362, 3311. |
| [14] | (a) Zhang, Z.; Huang, S.; Liu, W.; Zhao, L.-L.; Hu, C.; Yan, X. Green Synth. Catal 2023, 4, 300. |
| [14] | (b) McCallum, T. Green Synth. Catal 2023, 4, 10. |
| [14] | (c) Wang, H.; Xu, T. Chinese J. Org. Chem 2023, 43, 3328 (in Chinese). |
| [14] | (王贺盼, 徐涛, 有机化学, 2023, 43, 3328.) |
| [15] | (a) James, S. L.; Adams, C. J.; Bolm, C.; Braga, D.; Collier, P.; Fri??i?, T.; Grepioni, F.; Harris, K. D. M.; Hyett, G.; Jones, V.; Krebs, A.; Mack, J.; Maini, L.; Orpen, A. G.; Parkin, I. P.; Shearouse, W. C.; Steed, J. W.; Waddell, D. C. Chem. Soc. Rev 2012, 41, 413. |
| [15] | (b) Zhou, K.; Mao, Y.; Wu, F.; Lou, S.; Xu, D. Chinese J. Org. Chem. 2021, 41, 4623 (in Chinese). |
| [15] | (周琨, 毛羊杰, 吴峰伟, 娄绍杰, 许丹倩, 有机化学, 2021, 41, 4623.) |
| [15] | (c) Wang, H.; Ying, P.; Yu, J.; Su, W. Chinese J. Org. Chem. 2021, 41, 1897 (in Chinese). |
| [15] | (王浩, 应娉, 俞静波, 苏为科, 有机化学, 2021, 41, 1897.) |
| [16] | (a) Wang, G.-W.; Komatsu, K.; Murata, Y.; Shiro, M. Nature 1997, 387, 583. |
| [16] | (b) Wang, G.-W. Chin. J. Chem 2021, 39, 1797. |
| [16] | (c) Li, L.; Niu, C.; Wang, G.-W. Chin. J. Chem 2022, 40, 2539. |
| [17] | Koch, A.; Dufrois, Q.; Wirgenings, M.; G?rls, H.; Krieck, S.; Etienne, M.; Pohnert, G.; Westerhausen, M. Chem.-Eur. J. 2018, 24, 16840. |
| [18] | Pharmacopeia of the United States of America, 32nd revision, and the National Formulary, 27th ed., US Pharmacopeia, 2009. |
/
| 〈 |
|
〉 |