

葛根素纳米银合成及光热杀菌和促糖尿病感染伤口愈合作用研究
收稿日期: 2024-08-25
网络出版日期: 2024-10-22
基金资助
国家自然科学基金(22103007); 国家自然科学基金(82274353); 陕西省重点研发计划(2024SF-YBXM-517); 陕西省重点研发计划(2024SF-YBXM-426); 陕西省创新能力支撑计划-青年科技新星项目(2023KJXX-063); 中央高校基本科研业务费(300102124207); 陕西中医药大学2023年度科技创新人才体系建设计划(2023-LJRC-03); 秦药特色资源研究开发重点实验室开放课题项目(SUCM-QM202207)
The Fabrication of Silver Nanoparticles Using Puerarin and the Photothermal Sterilization and Diabetic Wound Healing Behavior
Received date: 2024-08-25
Online published: 2024-10-22
Supported by
National Natural Science Foundation of China(22103007); National Natural Science Foundation of China(82274353); Key Research and Development Plan of Shaanxi Province(2024SF-YBXM-517); Key Research and Development Plan of Shaanxi Province(2024SF-YBXM-426); Science and Technology Youth Stars Project of Shaanxi Province(2023KJXX-063); Fundamental Research Funds for the Central Universities, CHD(300102124207); Science and Technology Innovation Talent System Construction Plan of Shaanxi University of Chinese medicine(2023-LJRC-03); Open Project of State Key Laboratory of Research and Development of Characteristic Qin Medicine Resources(SUCM-QM202207)
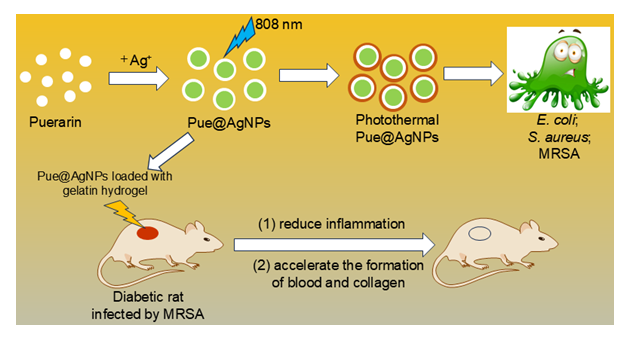
本工作直接使用天然药物分子葛根素作为还原剂和保护剂绿色制备了纳米银(Pue@AgNPs). Pue@AgNPs为近球形, 粒径主要分布在10~20 nm, 有少量聚集体, 表面吸附带负电荷物质, 为面心立方结构. Pue@AgNPs具有光热效应, 可捕获2,2'-联氮-二(3-乙基-苯并噻唑-6-磺酸)二铵盐自由基阳离子(ABTS•+)和1,1-二苯基-2-三硝基苯肼(DPPH)自由基, 被用作光热试剂和抗氧化剂. 对大肠杆菌(E. coli)、金黄色葡萄球菌(S. aureus)和耐甲氧西林金黄色葡萄球菌(MRSA)最小抑制浓度均为50.0 μg•mL−1; 可通过减轻伤口处炎症反应、加速新生血管和胶原纤维生成促进糖尿病大鼠伤口闭合.
王英辉 , 王雨辉 , 熊佳瑶 , 苏思琪 , 郝梦珂 , 魏思敏 . 葛根素纳米银合成及光热杀菌和促糖尿病感染伤口愈合作用研究[J]. 化学学报, 2024 , 82(11) : 1150 -1161 . DOI: 10.6023/A24080252
The complexity of plant extract incurs the uncertainty of capping and reducing agents in the biosynthesis of silver nanoparticles (AgNPs) leading to a poor reproducibility, which dramatically limits the application of this method. To overcome this problem, the puerarin (Pue), which is an isoflavone derived from kudzu roots and has strong biological activities, is directly used as the reducing and capping agents to prepare bioactive AgNPs (Pue@AgNPs). The influence of biosynthesis parameters like pH (Pue solution), concentration of both Pue and AgNO3 and incubation time were assessed to obtain Pue@AgNPs with superior bactericidal activity and photothermal effect. The as-prepared AgNPs are nearly spherical mainly existing in the monodispersed form with a small amount of aggregation, covering by anions (-44.0 mV) and revealing a high degree of stability (at least 15 days). It is conceived that the aggregation of Pue@AgNPs is responsible for absorbing near-infrared light (808 nm). The X-ray diffraction (XRD), transmission electron microscopy (TEM), high-resolution transmission electron microscopy (HRTEM) and selected area electron diffraction (SAED) studies show that Pue@AgNPs has a face-centered cubic (fcc) structure. The Pue@AgNPs show potent photothermal effect and antibacterial activity against Escherichia coli (E. coli), Staphylococcus aureus (S. aureus) and Methicillin-resistant Staphylococcus aureus (MRSA) under 808 nm laser radiation. The minimum inhibition concentration (MIC) values for E. coli, S. aureus and MRSA are 50.0 μg•mL−1. The internalization of Pue@AgNPs displays a variant trend suggesting the different mechanism of cell death. For E. coli, cell wall and intracellular damage may be responsible for cell death. However, for S. aureus and MRSA, the cell death may be rooted in oxidative stress or intracellular penetration. The Pue@AgNPs also exhibits superior antioxidation activity in trapping 2,2'-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) radical cation (ABTS•+) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical, and could accelerate diabetic wound contraction and healing within 21 days. The histological analysis showed that Pue@AgNPs could reduce inflammation and accelerate the formation of blood and collagen deposition.

| [1] | Guo, X. R.; Yin, Y. G.; Tan, Z. Q.; Liu, J. F.; Jiang, G. B. Acta Chim. Sinica 2018, 76, 387 (in Chinese). |
| [1] | (郭肖茹, 阴永光, 谭志强, 刘景富, 江桂斌, 化学学报, 2018, 76, 387.) |
| [2] | Chatterjee, S.; Lou, X. Y.; Liang, F.; Yang, Y. W. Coordin. Chem. Rev. 2022, 459, 214461. |
| [3] | Beck, F.; Loessl, M.; Baeumner, A. J. Microchim. Acta 2023, 190, 91. |
| [4] | Peng, H. C.; Chen, S.; Yan, B. Acta Chim. Sinica 2024, 82, 797 (in Chinese). |
| [4] | (彭红超, 陈胜, 阎斌, 化学学报, 2024, 82, 797.) |
| [5] | Loza, K.; Heggen, M.; Epple, M. Adv. Funct. Mater. 2020, 30, 1909260. |
| [6] | Oun, A. A.; Shankar, S.; Rhim, J. W. Crit. Rev. Food Sci. 2020, 60, 435. |
| [7] | Nguyen, N. T. T.; Nguyen, L. M.; Nguyen, T. T. T.; Liew, R. K.; Nguyen, D. T. C.; Tran, T. V. Sci. Total Environ. 2022, 827, 154160. |
| [8] | Wang, C.; Hong, T. T.; Cui, P. F.; Wang, J. H.; Xia, J. Adv. Drug Deliver. Rev. 2021, 175, 113818. |
| [9] | Xu, L.; Wang, Y. Y.; Huang, J.; Chen, C. Y.; Wang, Z. X.; Xie, H. Theranostics 2020, 10, 8996. |
| [10] | Mat'atková, O.; Michailidu, J.; Miskovská, A.; Kolouchová, I.; Masák, J.; Cejková, A. Biotechnol. Adv. 2022, 58, 107905. |
| [11] | Nie, P. H.; Zhao, Y.; Xu, H. Y. Ecotox. Environ. Safe. 2023, 253, 114636. |
| [12] | Godoy-Gallardo, M.; Eckhard, U.; Delgado, L. M.; Puente, Y.; Hoyos-Nogués, M.; Gil, F. J.; Perez, R. A. Bioact. Mater. 2021, 6, 4470. |
| [13] | Li, J.; Zhang, W.; Ji, W. H.; Wang, J. Q.; Wang, N. X.; Wu, W. X.; Wu, Q.; Hou, X. Y.; Hu, W. B.; Li, L. J. Mater. Chem. B 2021, 9, 7909. |
| [14] | Chen, Y. T.; Zhuo, M. P.; Wen, X. Y.; Chen, W. B.; Zhang, K. Q.; Li, M. D. Adv. Sci. 2023, 10, 2206830. |
| [15] | Zhao, L. P.; Zhang, X.; Wang, X. X.; Guan, X. W.; Zhang, W. F.; Ma, J. L. J. Nanobiotechnol. 2021, 19, 335. |
| [16] | Xu, H.; Han, P. B.; Qin, A. J.; Tang, B. Z. Acta Chim. Sinica 2023, 81, 1420 (in Chinese). |
| [16] | (徐赫, 韩鹏博, 秦安, 唐本忠, 化学学报, 2023, 81, 1420.) |
| [17] | Sun, H. T.; Zhang, Q.; Li, J. C.; Peng, S. J.; Wang, X. L.; Cai, R. Nano Today 2021, 37, 101073. |
| [18] | Zheng, B. D.; He, Q. X.; Li, X. S.; Yoon, J.; Huang, J. D. Coordin. Chem. Rev. 2021, 426, 213548. |
| [19] | Maleki, A.; He, J. H.; Bochani, S.; Nosrati, V.; Shahbazi, M. A.; Guo, B. L. ACS Nano 2021, 15, 18895. |
| [20] | Hao, S. Y.; Han, H. C.; Yang, Z. Y.; Chen, M. T.; Jiang, Y. Y.; Lu, G. X.; Dong, L.; Wen, H. L.; Li, H.; Liu, J. R.; Wu, L. L.; Wang, Z.; Wang, F. L. Nano-Micro Lett. 2022, 14, 178. |
| [21] | Huo, J. J.; Jia, Q. Y.; Huang, H.; Zhang, J.; Li, P.; Dong, X. C.; Huang, W. Chem. Soc. Rev. 2021, 50, 8762. |
| [22] | Mustapha, T.; Misni, N.; Ithnin, N. R.; Daskum, A. M.; Unyah, N. Z. Int. J. Env. Res. Pub. Heal. 2022, 19, 674. |
| [23] | Jadoun, S.; Arif, R.; Jangid, N. K.; Meena, R. K. Environ. Chem. Lett. 2021, 19, 355. |
| [24] | Mikhailova, E. O. J. Funct. Biomater. 2020, 11, 84. |
| [25] | Alharbi, N. S.; Alsubhi, N. S.; Felimban, A. I. J. Radiat. Res. Appl. Sci. 2022, 15, 109. |
| [26] | Rahuman, H. B. H.; Dhandapani, R.; Narayanan, S.; Palanivel, V.; Paramasivam, R.; Subbarayalu, R.; Thangavelu, S.; Muthupandian, S. IET Nanobiotechnol. 2022, 16, 115. |
| [27] | Moradi, F.; Sedaghat, S.; Moradi, O.; Salmanabadi, S. A. Inorg. Nano-Met. Chem. 2021, 51, 133. |
| [28] | Nayak, D.; Pradhan, S.; Ashe, S.; Rauta, P. R.; Nayak, B. J. Colloid Interf. Sci. 2015, 457, 329. |
| [29] | Zhang, Y. Q.; Cheng, X. F.; Zhang, Y. C.; Xue, X. H.; Fu, Y. Z. Colloid. Surface. A 2013, 423, 63. |
| [30] | Reddy, N. V.; Li, H. Z.; Hou, T. Y.; Bethu, M. S.; Ren, Z. Q.; Zhang, Z. J. Int. J. Nanomed. 2021, 16, 15. |
| [31] | He, Y.; Du, Z. Y.; Lv, H. B.; Jia, Q. F.; Tang, Z. K.; Zheng, X.; Zhang, K.; Zhao, F. H. Int. J. Nanomed. 2013, 8, 1809. |
| [32] | Rakib-Uz-Zaman, S. M.; Hoque Apu, E.; Muntasir, M. N.; Mowna, S. A.; Khanom, M. G.; Jahan, S. S.; Akter, N.; R. Khan, M. A.; Shuborna, N. S.; Shams, S. M.; Khan, K. Challenges 2022, 13, 18. |
| [33] | Anandan, M.; Poorani, G.; Boomi, P.; Varunkumar, K.; Anand, K.; Chuturgoon, A. A.; Saravanan, M.; Prabu, H. G. Process Biochem. 2019, 80, 80. |
| [34] | Wang, Y. H.; Wei, S. M.; Wang, K.; Wang, Z.; Duan, J. W.; Cui, L.; Zheng, H. Y.; Wang, Y.; Wang, S. S. RSC Adv. 2020, 10, 27173. |
| [35] | Wei, S.; Tang, Z.; Li, H.; Zhang, K.; Song, Z. Chin. Tradit. Herb. Drugs 2019, 50, 52 (in Chinese). |
| [35] | (魏思敏, 唐志书, 李慧敏, 张可可, 宋忠兴, 中草药, 2019, 50, 52.) |
| [36] | Wei, S. M.; Wang, Y. H.; Tang, Z. S.; Su, R.; Hu, J. H.; Guo, H.; Li, C.; Jiang, J. T.; Song, Z. X. Chem. J. Chin. Univ. 2020, 41, 1391 (in Chinese). |
| [36] | (魏思敏, 王英辉, 唐志书, 苏瑞, 胡锦航, 郭惠, 李琛, 蒋金涛, 宋忠兴, 高等学校化学学报, 2020, 41, 1391.) |
| [37] | Wang, Y. H.; Wei, S. M. ACS Omega 2022, 7, 1494. |
| [38] | Wei, S. M.; Wang, Y. H.; Tang, Z. S.; Hu, J. H.; Su, R.; Lin, J. J.; Zhou, T.; Guo, H.; Wang, N.; Xu, R. R. New J. Chem. 2020, 44, 9304. |
| [39] | Wei, S. M.; Wang, Y. H.; Tang, Z. S.; Xu, H. B.; Wang, Z.; Yang, T.; Zou, T. Y. RSC Adv. 2021, 11, 1411. |
| [40] | Wei, S.; Wang, Y.; Tang, Z.; Wang, Z.; Zhang, Z.; Su, R.; Jin, R.; Song, Z. Chin. Tradit. Herb. Drugs 2020, 51, 4169 (in Chinese). |
| [40] | (魏思敏, 王英辉, 唐志书, 王哲, 张珍, 苏瑞, 靳如意, 宋忠兴, 中草药, 2020, 51, 4169.) |
| [41] | Wang, Y.; Zou, T.; Su, R.; Wei, S. Chin. Tradit. Herb. Drugs 2022, 53, 1964 (in Chinese). |
| [41] | (王英辉, 邹太艳, 苏瑞, 魏思敏, 中草药, 2022, 53, 1964.) |
| [42] | Wei, S. M.; Hao, M. K.; Tang, Z. S.; Zhou, T.; Zhao, F.; Wang, Y. H. RSC Adv. 2022, 12, 36115. |
| [43] | Wei, D. W.; Sun, W. Y.; Qian, W. P.; Ye, Y. Z.; Ma, X. Y. Carbohyd. Res. 2009, 344, 2375. |
| [44] | De Matos, R. A.; Courrol, L. C. Amino Acids 2017, 49, 379. |
| [45] | Zeng, X. L.; Chen, B. H.; Wang, L. P.; Sun, Y. X.; Jin, Z.; Liu, X. Y.; Ouyang, L. P.; Liao, Y. Bioact. Mater. 2023, 19, 653. |
| [46] | Han, R. M.; Tian, Y. X.; Liu, Y.; Chen, C. H.; Ai, X. C.; Zhang, J. P.; Skibsted, L. H. J. Agric. Food Chem. 2009, 57, 3780. |
| [47] | Menichetti, A.; Mavridi-Printezi, A.; Mordini, D.; Montalti, M. J. Funct. Biomater. 2023, 14, 244. |
| [48] | Li, X. S.; Lovell, J. F.; Yoon, J.; Chen, X. Y. Nat. Rev. Clin. Oncol. 2020, 17, 657. |
| [49] | Kumari, M.; Shukla, S.; Pandey, S.; Giri, V. P.; Bhatia, A.; Tripathi, T.; Kakkar, P.; Nautiyal, C. S.; Mishra, A. ACS Appl. Mater. Interfaces 2017, 9, 4519. |
| [50] | Hao, M.; Wei, S.; Su, S.; Tang, Z.; Wang, Y. ACS Appl. Mater. Interfaces 2024, 16, 24221. |
/
| 〈 |
|
〉 |