

原位电势表征揭示聚合物固态锂电池界面降解机理
收稿日期: 2024-09-09
网络出版日期: 2024-11-05
基金资助
苏州市科技项目(SSD2023005)
In-situ Potential Characterization Reveals the Interface Degradation Mechanism of Solid-state Polymer Lithium Batteries
Received date: 2024-09-09
Online published: 2024-11-05
Supported by
Suzhou Science and Technology Program(SSD2023005)
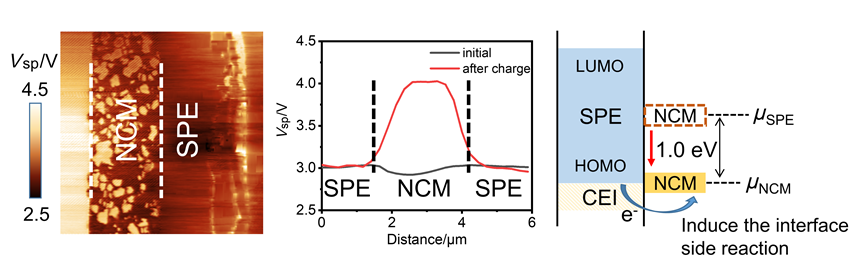
聚合物固态锂金属电池具有较高的能量密度和安全性, 十分具有发展前景. 然而, 该电池在高电压体系下的循环稳定性较差, 了解高电压体系下电池性能衰减的机理至关重要. 本工作使用扫描开尔文探针力显微镜对LiFePO4/LiNi0.6Co0.2Mn0.2O2 (NCM)正极与聚离子液体基聚合物电解质的界面电势变化进行原位表征分析. 结果表明高电压的NCM正极-电解质界面能级差由–0.1 eV上升到1.0 eV, 容易导致电解质优先失去电子而发生界面降解副反应, 从而引起正极-电解质界面结构性质变化, 增大了界面阻抗, 使电池循环性能显著下降. 因此, 该原位表征技术有助于提升电解质的高电压稳定性, 为发展性能优异的高电压聚合物固态锂电池提供指导帮助.
陈博文 , 徐克 , 陈琪 , 陈立桅 . 原位电势表征揭示聚合物固态锂电池界面降解机理[J]. 化学学报, 2024 , 82(12) : 1209 -1215 . DOI: 10.6023/A24090267
Solid-state polymer electrolyte lithium metal batteries (SPE-LIBs) with high energy density and safety have been widely considered as the next generation of lithium batteries. In recent years, the ion transport mechanism of SPE has been extensively studied, resulting in a significant increase in its ionic conductivity and lithium transference number. However, SPE has poor cycling stability when used with high-voltage cathodes, limiting its further development. Therefore, it is important to understand the mechanism of battery performance degradation in high-voltage systems. In this work, first, the SPE was prepared by photopolymerization using 1-butyl-3-vinylimidazolium bis(trifluoromethylsulfonyl) imide, vinylethylene carbonate and poly(ethylene glycol) diacrylate. The LiFePO4(LFP)||SPE||Li battery was prepared by the in-situ polymerization method. This battery has an initial discharge specific capacity of 159.6 mAh•g-1 at room temperature, and the capacity retention rate reaches 95% after 145 cycles, indicating that the cell has a low interface impedance and the electrolyte has a high ionic conductivity. Then, in-situ Scanning Kelvin Probe Force Microscopy was used to characterize the interfacial potential of the cross-sections of batteries with two different cathodes, which were prepared by argon ion beam polishing, during charging. Compared with the LFP cathode, the LiNi0.6Co0.2Mn0.2O2 (NCM) cathode with higher electrochemical reaction potential has a greater potential difference between the cathode and the electrolyte after charging. As a result, it indicates that the energy level of high-voltage cathode materials changes greatly during the charging process, which makes the electrolyte materials more susceptible to losing electrons preferentially and cause degradation side reactions. Combined with electrochemical impedance spectroscopy and laser confocal Raman spectroscopy to characterize the interfacial structure of the SPE-LIB. It found that side reactions would destroy the structure of the cathode electrolyte interphase, resulting in a significant increase in the interfacial impedance and the attenuation of the battery capacity. The electrolyte degradation mechanism of SPE-LIBs under high-voltage systems was revealed through in-situ characterization, which provided guidance for improving the cycling stability with high-voltage cathodes.

| [1] | Li, M.; Lu, J.; Chen, Z.; Amine, K. Adv. Mater. 2018, 30, 1800561. |
| [2] | Li, H. Joule 2019, 3, 911. |
| [3] | Grey, C. P.; Hall, D. S. Nat. Commun. 2020, 11, 6279. |
| [4] | Cheng, X. B.; Zhang, R.; Zhao, C. Z.; Zhang, Q. Chem. Rev. 2017, 117, 10403. |
| [5] | Lin, D.; Liu, Y.; Cui, Y. Nat. Nanotechnol. 2017, 12, 194. |
| [6] | Tao, M. M.; Chen, J. N.; Lin, H. X.; Zhou, Y. A.; Zhao, D. H.; Shan, P. Z.; Jin, Y. T.; Yang, Y. J. Energy Chem. 2024, 96, 226. |
| [7] | Wang, Q. Y.; Liu, B.; Shen, Y. H.; Wu, J. K.; Zhao, Z. Q.; Zhong, C.; Hu, W. B. Adv. Sci. 2021, 8, 2101111. |
| [8] | Manthiram, A.; Yu, X. W.; Wang, S. F. Nat. Rev. Mater. 2017, 2, 16103. |
| [9] | Kalnaus, S.; Dudney, N. J.; Westover, A. S.; Herbert, E.; Hackney, S. Science 2023, 381, 1300. |
| [10] | Bocharova, V.; Sokolov, A. P. Macromolecules 2020, 53, 4141. |
| [11] | Song, Z.; Chen, F.; Martinez-Iba?ez, M.; Feng, W.; Forsyth, M.; Zhou, Z.; Armand, M.; Zhang, H. Nat. Commun. 2023, 14, 4884. |
| [12] | Tian, S. W.; Zhou, L. X.; Zhang, B. Q.; Zhang, J. J.; Du, X. F.; Zhang, H.; Hu, S. J.; Yuan, Z. X.; Han, P. X.; Li, S. L.; Zhao, W.; Zhou, X. H.; Cui, G. L. Acta Chim. Sinica 2022, 80, 1410. (in Chinese) |
| [12] | (田宋炜, 周丽雪, 张秉乾, 张建军, 杜晓璠, 张浩, 胡思伽, 苑志祥, 韩鹏献, 李素丽, 赵伟, 周新红, 崔光磊, 化学学报, 2022, 80, 1410.) |
| [13] | Atik, J.; Diddens, D.; Thienenkamp, J. H.; Brunklaus, G.; Winter, M.; Paillard, E. Angew. Chem.-Int. Ed. 2021, 60, 11919. |
| [14] | Zou, Z.; Li, Y.; Lu, Z.; Wang, D.; Cui, Y.; Guo, B.; Li, Y.; Liang, X.; Feng, J.; Li, H.; Nan, C. W.; Armand, M.; Chen, L.; Xu, K.; Shi, S. Chem. Rev. 2020, 120, 4169. |
| [15] | Wang, H.; Song, J.; Zhang, K.; Fang, Q.; Zuo, Y.; Yang, T.; Yang, Y.; Gao, C.; Wang, X.; Pang, Q.; Xia, D. Energy Environ. Sci. 2022, 15, 5149. |
| [16] | Liu, J.; Yang, Z.; Liu, W.; Yang, Z.; Chen, S.; Li, R.; Huang, T.; Liu, H. Sci. Sin. Chim. 2023, 53, 1277. (in Chinese) |
| [16] | (刘嘉星, 杨智昊, 刘维捷, 阳征斐, 陈苏越, 李若兰, 黄铁骑, 刘洪涛, 中国科学: 化学, 2023, 53, 1277.) |
| [17] | Zhang, H.; Li, C.; Piszcz, M.; Coya, E.; Rojo, T.; Rodriguez-Martinez, L. M.; Armand, M.; Zhou, Z. Chem. Soc. Rev. 2017, 46, 797. |
| [18] | Xue, G. Y.; Li, J.; Chen, J. C.; Chen, D. Q.; Hu, C. J.; Tang, L. F.; Chen, B. W.; Yi, R. W.; Shen, Y. B.; Chen, L. W. Acta Phys.-Chim. Sin. 2023, 39, 2205012. |
| [19] | Caba?ero Martínez, M. A.; Boaretto, N.; Naylor, A. J.; Alcaide, F.; Salian, G. D.; Palombarini, F.; Ayerbe, E.; Borras, M.; Casas‐ Cabanas, M. Adv. Energy Mater. 2022, 12, 2201264. |
| [20] | Gupta, H.; Singh, S. K.; Srivastava, N.; Meghnani, D.; Tiwari, R. K.; Mishra, R.; Patel, A.; Tiwari, A.; Saroj, A. L.; Singh, R. K. ACS Appl. Energ. Mater. 2021, 4, 13878. |
| [21] | Liu, J. G.; Li, B. H.; Cao, J. H.; Xing, X.; Cui, G. J. Energy Chem. 2024, 91, 73. |
| [22] | Vijayakumar, V.; Anothumakkool, B.; Kurungot, S.; Winter, M.; Nair, J. R. Energy Environ. Sci. 2021, 14, 2708. |
| [23] | Liu, T. T.; Zhang, J. J.; Han, W.; Zhang, J. N.; Ding, G. L.; Dong, S. M.; Cui, G. L. J. Electrochem. Soc. 2020, 167, 070527. |
| [24] | Melitz, W.; Shen, J.; Kummel, A. C.; Lee, S. Surf. Sci. Rep. 2011, 66, 1. |
| [25] | Chen, X.; Lai, J. Q.; Shen, Y. B.; Chen, Q.; Chen, L. W. Adv. Mater. 2018, 30, 1802490. |
| [26] | Wu, F.; Maier, J.; Yu, Y. Chem. Soc. Rev. 2020, 49, 1569. |
| [27] | Goodenough, J. B.; Kim, Y. Chem. Mater. 2010, 22, 587. |
| [28] | Choi, W.; Shin, H. C.; Kim, J. M.; Choi, J. Y.; Yoon, W. S. J. Electrochem. Sci. Technol. 2020, 11, 1. |
/
| 〈 |
|
〉 |