

果糖一步法制备5-甲氧基甲基-2-呋喃甲醛及树脂催化剂再生方法探究
收稿日期: 2024-08-21
网络出版日期: 2024-11-06
基金资助
辽宁振兴人才计划(XLYC1907029)
One-step Preparation of 5-(Methoxymethyl)-2-furaldehyde from Fructose and Regeneration of Resin Catalyst
Received date: 2024-08-21
Online published: 2024-11-06
Supported by
Liaoning Revitalization Talents Program(XLYC1907029)
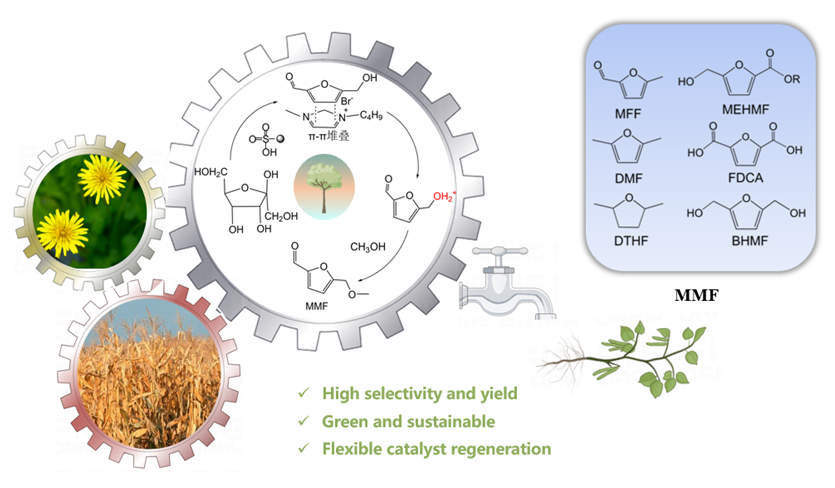
5-甲氧基甲基-2-呋喃甲醛(MMF)是一种重要的生物基平台分子, 可作为燃料添加剂和用于制备多种化合物. 本工作利用一系列商业强酸性树脂催化剂, 以离子液体和甲醇为共溶剂, 实现了从果糖一步法向MMF的转化. 离子液体与中间产物5-羟甲基糠醛(HMF)之间具有强相互作用, 可以减少HMF自身缩合等副反应的发生, 提高MMF的产率. 在最佳反应条件下, 果糖的转化近乎完全, MMF的产率达到67.7%, HMF与MMF的总产率达到接近75%. 使用BET表面分析技术、红外光谱、元素分析及酸碱滴定技术对使用前后的催化剂进行表征, 证实了催化剂失活的可能机制是胡敏覆盖催化剂活性位点、树脂催化剂与离子液体之间的离子交换及催化剂酸性位点的微量脱落. 探索出合适的催化剂再生方案, 实现了催化剂的稳定循环. 此外, 本催化体系还可以将其它碳水化合物中的果糖组分高效转化为MMF, 为生物质转化制备MMF的产业化奠定了一定的研究基础.
鞠嘉浩 , 徐吉磊 , 王康军 , 黄家辉 . 果糖一步法制备5-甲氧基甲基-2-呋喃甲醛及树脂催化剂再生方法探究[J]. 化学学报, 2024 , 82(12) : 1216 -1225 . DOI: 10.6023/A24080246
With the excessive use of fossil energy, energy and environmental problems have become increasingly prominent, and the search for new energy sources is imminent. Biomass is renewable, widely distributed, carbon neutral, and can be used to produce a variety of chemicals, so it has attracted wide attention from the scientific community and the business community. 5-Hydroxymethylfurfural (HMF) is an important biomass-based platform molecule that can be used to synthesize a variety of chemicals and fuels. It is a key intermediate for the synthesis of bio-based polyester monomer 2,5-Furandicarboxylic acid (FDCA). However, HMF is very active and heat-sensitive, and can self-polymerize at room temperature to form by-products such as humin. 5-(Methoxymethyl)-2-furaldehyde (MMF) is a new bio-based platform molecule. Its structure is similar with HMF, but its properties are more stable. It can be separated from the reaction system by simple distillation, and the separation cost is much lower than that of HMF, which is expected to replace HMF as a key platform compound for the product of bio-based furan chemicals. Therefore, the direct conversion of bio-based raw materials into MMF has very important research value and industrial significance. In this paper, a series of resin catalysts were used to realize the one-step conversion from fructose to MMF using ionic liquids and methanol as cosolvent. Under the best conditions, about 67.7% yield of MMF was obtained in the present of DA-330, which is higher than related research. However, the activity of DA-330 resin decreases very quickly in the reaction system. The yield of MMF was about 10% during the second experiment, which was much lower than that of the first time. After a series of characterizations, such as Brunner-Emmet-Teller measurements, Fourier transform infrared spectroscopy, acid base titration and elemental analysis, it was determined that the deactivation of the resin catalyst was due to the coverage of humin, ion exchange between the resin and ionic liquid, and the loss of acid sites of resin. After hydrogen peroxide oxidation, H+ ion exchange, the activity of the resin catalyst was restored to the level of the new catalyst, and the yield of MMF did not significantly decrease after recycled four times.

| [1] | Huber, G. W.; Ibarra, S. Acc. Chem. Res. 2006, 106, 4044. |
| [2] | Srirangan, K.; Akawi, L.; Moo-Young, M. Appl. Energy 2012, 100, 172. |
| [3] | Guo, J.-R.; Zhang, S.-Y.; He, Y.-H.; Ren, S.-X. Acta Chim. Sinica 2024, 82, 242. (in Chinese) |
| [3] | (郭建荣, 张书玉, 贺宇辉, 任世学, 化学学报, 2024, 82, 242.) |
| [4] | Gao, J.-B.; Lu, Y.-Q.; Zhang, H.; Gao, L.-Z.; Xiong, X.-Q. Chinese J. Org. Chem. 2024, 44, 2732. (in Chinese) |
| [4] | (高晋斌, 陆颖琪, 张辉, 高立柱, 熊兴泉, 有机化学, 2024, 44, 2732.) |
| [5] | Fu, J.; Lin, G.-B.; Fan, H.-A.; Chen, C.; Li, B.-L.; Zhan, Y.; Zhao, X.-Z. ACS Catal. 2024, 14, 1862. |
| [6] | Li, N.; Zong, M.-H.; Li, Y.; Shi, S.-S. ACS Sustainable Chem. Eng. 2020, 8, 1437. |
| [7] | Pfab, E.; Filiciotto, L.; Romero, A. A.; Luque, R. Ind. Eng. Chem. Res. 2019, 58, 16065. |
| [8] | Bicker, M.; Kaiser, D.; Ott, L.; Vogel, H. J. Supercrit. Fluids 2005, 36, 118. |
| [9] | Balakrishnan, M.; Sacia, E. R.; Bell, A. T. Green Chem. 2012, 14, 1626. |
| [10] | Zhang, Z.-H.; Wang, Y.-M.; Fang, Z.-F.; Liu, B. ChemPlusChem 2014, 79, 233. |
| [11] | Yuan, Z.-L.; Zhang, Z.-H.; Zheng, J.-D.; Lin, J.-T. Fuel 2015, 150, 236. |
| [12] | Che, P.-H.; Lu, F.; Zhang, J.-Z.; Huang, Y.-Z.; Nie, X.; Gao, J. Bioresour. Technol. 2012, 119, 433. |
| [13] | Yin, S.; Sun, J.; Liu, B.; Zhang, Z.-H. J. Mater. Chem. A 2015, 3, 4992. |
| [14] | Liu, B.; Zhang, Z.-H. RSC Adv. 2013, 3, 12313. |
| [15] | Lai, L.-K.; Zhang, Y.-G. ChemSusChem 2011, 4, 1745. |
| [16] | Kraus, G. A.; Guney, T. Green Chem. 2012, 14, 1593. |
| [17] | Nie, Y.-F.; Hou, Q.-D.; Qian, H.-L.; Bai, X.-Y.; Xia, T.-L.; Yu, G.-J.; Ju, M.-T. Renewable Energy. 2022, 192, 279. |
| [18] | Dai, J.-H.; Du, Z.-T.; Cao, Q.-Y.; Yang, R.-H.; Yang, D.-L.; Li, J.-L. Mol. Catal. 2024, 599, 114112. |
| [19] | Guo, H.-X.; Qi, X.-H.; Shen, F. ACS Sustainable Chem. 2022, 10, 9002. |
| [20] | Guranix, G. J. M.; Dautzenberg, F.CN101400666A (B) 2007 [Chem. Abstr. 2007, 147, 367433]. |
| [21] | Shibasaki-Kitakawa, N.; Honda, H.; Kuribayashi, H. Bioresour. Technol. 2007, 98, 416. |
| [22] | Figueira, M.; Reig, M.; de Labastida, M. F. J. Environ. Manage. 2022, 314, 114984. |
| [23] | Li, S.-S.; Li, N.; Wang, W.; Li, G.-Y.; Chen, F. Green Chem. 2016, 18, 1218. |
| [24] | Zhu, H.; Cao, Q.; Li, C.-H.; Mu, X.-D. Carbohydr. Res. 2011, 346, 2016. |
| [25] | Fu, J.; Yang, H.; Zhou, F.; Li, J.; Ma, H.-X.; Chen, K.-Q.; Lu, X.-Y. Ind. Eng. Chem. Res. 2020, 59, 4905. |
| [26] | Gao, F.; Bai, R.; Ferlin, F.; Vaccaro, L.; Li, M.; Gu, Y. Green Chem. 2020, 22, 6240. |
| [27] | Anushree, S.; Ramsingh, K.; Kanti, D. M.; Kumar, S. Spectrochim. Acta, Part A 2021, 262, 120144. |
| [28] | Xiang, Y.-P.; Wen, S.; Tian, Y.; Zhao, K.-Y.; Guo, D.-W.; Cheng, F.; Xu, Q.; Liu, X.-X.; Yin, D.-L. RSC Adv. 2011, 11, 3585. |
| [29] | Kashbora, M. M.; Sutarmaa, D.; Railtona, J.; Liu, X. Appl. Catal., A 2022, 642, 118689. |
| [30] | Hoang, T. M. C.; Lefferts, L.; Seshan, K. ChemSusChem 2013, 6, 1651. |
| [31] | Yu, Y.-M. Journal of Shengli College China University of Petroleum. 2007, 4, 21. (in Chinese) |
| [31] | (于颖敏, 中国石油大学胜利学院学报, 2007, 4, 21.) |
| [32] | Ren, Y.-S.; Liu, B.; Zhang, Z.-H.; Lin, J. T. J. Ind. Eng. Chem. 2015, 21, 1127. |
| [33] | Li, R.-P.; Wang, Y.-P.; Zhao, Y.-F.; Zhang, F.-T.; Zeng, W.; Tang, M.-H.; Xiang, J.-F.; Zhang, X.-Y.; Han, B.-X.; Liu, Z.-M. ACS Sustainable Chem. Eng. 2021, 9, 14216. |
/
| 〈 |
|
〉 |