

一价铜催化的酮或酮亚胺的不对称烯丙基化反应研究进展
收稿日期: 2024-10-11
网络出版日期: 2024-11-26
基金资助
国家自然科学基金(22271302); 山西省自然科学基金(202303021211189)
Research Progress on Copper(I)-Catalyzed Asymmetric Allylation of Ketones or Ketimines
Received date: 2024-10-11
Online published: 2024-11-26
Supported by
National Natural Science Foundation of China(22271302); Natural Science Foundation of Shanxi Province(202303021211189)
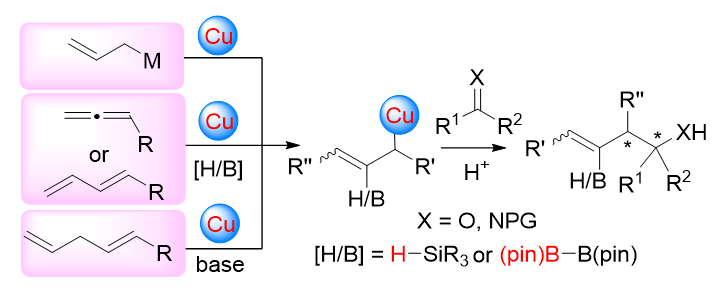
手性高烯丙基叔醇和相应的高烯丙基胺结构单元广泛存在于天然产物和药物分子中. 因此, 其不对称合成受到合成化学家们的重视. 过渡金属催化的酮或酮亚胺的不对称烯丙基化反应具有优秀的原子经济性和步骤经济性, 是合成手性高烯丙基叔醇和相应的高烯丙基胺最直接有效的方法之一. 此综述着重介绍了一价铜催化的酮或酮亚胺不对称烯丙基化反应的研究进展. 根据生成烯丙基一价铜物种的不同方式, 将反应分为转金属化反应、 三组分偶联反应和质子转移反应. 另外, 对一些具有代表性的反应机理和产物的合成应用做了简要介绍, 并对该领域的发展进行了展望.
李晖 , 殷亮 . 一价铜催化的酮或酮亚胺的不对称烯丙基化反应研究进展[J]. 化学学报, 2024 , 82(12) : 1274 -1288 . DOI: 10.6023/A24100300
Chiral tertiary homoallylic alcohol and chiral α,α-disubstituted homoallylic amine scaffolds are ubiquitous in numerous bioactive natural products and pharmaceutically relevant molecules. Thus, asymmetric synthesis of these compounds has attracted an increasing attention from synthetic chemists. Compared to traditional methods, transition-metal-catalyzed asymmetric allylation of ketones or ketimines serves as a powerful methodology for constructing these compounds due to its excellent atom- and step-economy. Recent progress on copper(I)-catalyzed asymmetric allylation of ketones or ketimines is summarized. Based on the strategies for the generation of allyl-copper(I) species in situ, this review is divided into three sections: reactions through transmetalation, three-component coupling reactions, and proton-transfer reactions. The mechanisms and potential applications of some representative strategies are also included. Finally, the future developments in this field are outlooked.

| [1] | (a) Mizui, Y.; Sakai, T.; Iwata, M.; Uenaka, T.; Okamoto, K.; Shimizu, H.; Yamori, T.; Yoshimatsu, K.; Asada, M. J. Antibiot. 2004, 57, 188. |
| [1] | (b) Paterson, I.; Dalby, S. M.; Roberts, J. C.; Naylor, G. J.; Guzmán, E. A.; Isbrucker, R.; Pitts, T. P.; Linley, P.; Divlianska, D.; Reed, J. K.; Wright, A. E. Angew. Chem., Int. Ed. 2011, 50, 3219. |
| [1] | (c) Friestad, G. K.; Mathies, A. K. Tetrahedron 2007, 63, 2541. |
| [2] | (a) Pu, L.; Yu, H.-B. Chem. Rev. 2001, 101, 757. |
| [2] | (b) Shibasaki, M.; Kanai, M. Chem. Rev. 2008, 108, 2853. |
| [3] | (a) Read, J. A.; Woerpel, K. A. J. Org. Chem. 2017, 82, 2300. |
| [3] | (b) Bartolo, N. D.; Woerpel, K. A. J. Org. Chem. 2018, 83, 10197. |
| [4] | Cervera-Padrell, A. E.; Nielsen, J. P.; Pedersen, M. J.; Christensen, K. M.; Mortensen, A. R.; Skovby, T.; Dam-Johansen, K.; Kiil, S.; Gernaey, K. V. Org. Process Res. Dev. 2012, 16, 901. |
| [5] | Yamasaki, S.; Fujii, K.; Wada, R.; Kanai, M.; Shibasaki, M. J. Am. Chem. Soc. 2002, 124, 6536. |
| [6] | Wada, R.; Oisaki, K.; Kanai, M.; Shibasaki, M. J. Am. Chem. Soc. 2004, 126, 8910. |
| [7] | Kanai, M.; Wada, P.; Shibuguchi, T.; Shibasaki, M. Pure Appl. Chem. 2008, 80, 1055. |
| [8] | Shi, S.-L.; Xu, L.-W.; Oisaki, K.; Kanai, M.; Shibasaki, M. J. Am. Chem. Soc. 2010, 132, 6638. |
| [9] | Motoki, R.; Tomita, D.; Kanai, M.; Shibasaki, M. Tetrahedron Lett. 2006, 47, 8083. |
| [10] | Iwamoto, H.; Hayashi, Y.; Ozawa, Y.; Ito, H. ACS Catal. 2020, 10, 2471. |
| [11] | Zanghi, J. M.; Meek, S. J. Angew. Chem., Int. Ed. 2020, 59, 8451. |
| [12] | Sun, B.; Ruan, L.-X.; Zhao, R.; Zhang, J.; Niu, R.; Luo, Q.; Zhang, Y.; Gao, L.; Shi, S.-L. Nat. Synth. 2024, 3, 1091. |
| [13] | Wada, R.; Shibuguchi, T.; Makino, S.; Oisaki, K.; Kanai, M.; Shibasaki, M. J. Am. Chem. Soc. 2006, 128, 7687. |
| [14] | Tsai, E. Y.; Liu, R. Y.; Yang, Y.; Buchwald, S. L. J. Am. Chem. Soc. 2018, 140, 2007. |
| [15] | Liu, R. Y.; Zhou, Y.; Yang, Y.; Buchwald, S. L. J. Am. Chem. Soc. 2019, 141, 2251. |
| [16] | Xu, M.; Lu, Q.; Gong, B.; Ti, W.; Lin, A.; Yao, H.; Gao, S. Angew. Chem., Int. Ed. 2023, 62, e202311540. |
| [17] | (a) Gupta, P.; Mahajan, N. New J. Chem. 2018, 42, 12296. |
| [17] | (b) Mushtaq, S.; Abbasi, B. H.; Uzair, B.; Abbasi, R. EXCLI Journal 2018, 17, 420. |
| [18] | Klake, R. K.; Edwards, M. D.; Sieber, J. D. Org. Lett. 2021, 23, 6444. |
| [19] | Collins, S.; Sieber, J. D. Org. Lett. 2023, 25, 1425. |
| [20] | Min, L.; Han, J.-C.; Zhang, W.; Gu, C.-C.; Zou, Y.-P.; Li, C.-C. Chem. Rev. 2023, 123, 4934. |
| [21] | Zhou, M.; Lin, Y.; Chen, X.-X.; Xu, G.; Chung, L. W.; Tang, W. Angew. Chem., Int. Ed. 2023, 62, e202300334. |
| [22] | Jiang, N.; Liu, P.-Z.; Pan, Z.-Z.; Wang, S.-Q.; Peng, Q.; Yin, L. Angew. Chem., Int. Ed. 2024, 63, e202402195. |
| [23] | Yang, Y.; Perry, I. B.; Lu, G.; Liu, P.; Buchwald, S. L. Science 2016, 353, 144. |
| [24] | Li, C.; Liu, R. Y.; Jesikiewicz, L. T.; Yang, Y.; Liu, P.; Buchwald, S. L. J. Am. Chem. Soc. 2019, 141, 5062. |
| [25] | Fu, B.; Yuan, X.; Li, Y.; Wang, Y.; Zhang, Q.; Xiong, T.; Zhang, Q. Org. Lett. 2019, 21, 3576. |
| [26] | (a) McManus, H. A.; Fleming, M. J.; Lautens, M. Angew. Chem., Int. Ed. 2007, 46, 433. |
| [26] | (b) Perrone, R.; Berardi, F.; Colabufo, N. A.; Leopoldo, M.; Tortorella, V.; Fiorentini, F.; Olgiati, V.; Ghiglieri, A.; Govoni, S. J. Med. Chem. 1995, 38, 942. |
| [27] | Acharyya, R. K.; Kim, S.; Park, Y.; Han, J. T.; Yun, J. Org. Lett. 2020, 22, 7897. |
| [28] | Zhu, J.; Rahim, F.; Zhou, P.; Zhang, A.; Malcolmson, S. J. J. Am. Chem. Soc. 2024, 146, 20270. |
| [29] | (a) Tewes, B.; Frehland, B.; Schepmann, D.; Robaa, D.; Uengwetwanit, T.; Gaube, F.; Winckler, T.; Sippl, W.; Wu?nsch, B. J. Med. Chem. 2015, 58, 6293. |
| [29] | (b) Novoa, A.; Van Dorpe, S.; Wynendaele, E.; Spetea, M.; Bracke, N.; Stalmans, S.; Betti, C.; Chung, N. N.; Lemieux, C.; Zuegg, J.; Cooper, M. A.; Tourwé, D.; De Spiegeleer, B.; Schiller, P. W.; Ballet, S. J. Med. Chem. 2012, 55, 9549. |
| [30] | Li, D.; Park, Y.; Yoon, W.; Yun, H.; Yun, J. Org. Lett. 2019, 21, 9699. |
| [31] | Deng, X.-H.; Jiang, J.-X.; Jiang, Q.; Yang, T.; Chen, B.; He, L.; Chu, W.-D.; He, C.-Y.; Liu, Q.-Z. Org. Lett. 2022, 24, 4586. |
| [32] | Meng, F.; Jang, H.; Jung, B.; Hoveyda, A. H. Angew. Chem., Int. Ed. 2013, 52, 5046. |
| [33] | Zhao, Y.-S.; Tang, X.-Q.; Tao, J.-C.; Tian, P.; Lin, G.-Q. Org. Biomol. Chem. 2016, 14, 4400. |
| [34] | (a) Yeung, K.; Talbot, F. J. T.; Howell, G. P.; Pulis, A. P.; Procter, D. J. ACS Catal. 2019, 9, 1655. |
| [34] | (b) Deng, H.; Dong, Y.; Yu, S.; Yang, F.; Han, S.; Wu, J.; Liang, B.; Guo, H.; Zhang, C. Org. Lett. 2021, 23, 4431. |
| [34] | (c) Ashraf, M. A.; Tambe, S. D.; Cho, E. J. Bull. Korean Chem. Soc. 2021, 42, 683. |
| [35] | Jang, H.; Romiti, F.; Torker, S.; Hoveyda, A. H. Nat. Chem. 2017, 9, 1269. |
| [36] | Zhao, C.-Y.; Zheng, H.; Ji, D.-W.; Min, X.-T.; Hu, Y.-C.; Chen, Q.-A. Cell Rep. Phys. Sci. 2020, 1, 100067. |
| [37] | Liu, X.; Shi, S. Chin. J. Org. Chem. 2024, 44, 1884. |
| [38] | Feng, J.-J.; Xu, Y.; Oestreich, M. Chem. Sci. 2019, 10, 9679. |
| [39] | Yoon, W. S.; Han, J. T.; Yun, J. Adv. Synth. Catal. 2021, 363, 4953. |
| [40] | Li, D.; Park, Y.; Yun, J. Org. Lett. 2018, 20, 7526. |
| [41] | Yazaki, R.; Kumagai, N.; Shibasaki, M. J. Am. Chem. Soc. 2010, 132, 5522. |
| [42] | Wei, X.-F.; Xie, X.-W.; Shimizu, Y.; Kanai, M. J. Am. Chem. Soc. 2017, 139, 4647. |
| [43] | Zhong, F.; Pan, Z.-Z.; Zhou, S.-W.; Zhang, H.-J.; Yin, L. J. Am. Chem. Soc. 2021, 143, 4556. |
| [44] | Liu, J.; Su, B.; Chen, M. Org. Lett. 2021, 23, 6035. |
| [45] | Pan, Z.-Z.; Li, J.-H.; Tian, H.; Yin, L. Angew. Chem., Int. Ed. 2024, 63, e202315293. |
| [46] | Pan, Z.-Z.; Pan, D.; Li, J.-H.; Xue, X.-S.; Yin, L. J. Am. Chem. Soc. 2023, 145, 1749. |
| [47] | (a) Fu, Z.; Xu, J.; Zhu, T.; Leong, W. W. Y.; Chi, Y. R. Nat. Chem. 2013, 5, 835. |
| [47] | (b) Xie, Y.; Yu, C.; Li, T.; Tu, S.; Yao, C. Chem. Eur. J. 2015, 21, 5355. |
| [47] | (c) Ma, J.; Rosales, A. R.; Huang, X.; Harms, K.; Riedel, R.; Wiest, O.; Meggers, E. J. Am. Chem. Soc. 2017, 139, 17245. |
/
| 〈 |
|
〉 |