

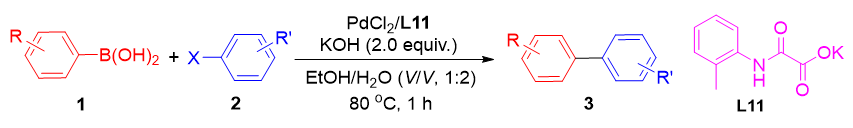
草酰单胺配体促进的低剂量钯催化水相Suzuki偶联反应
收稿日期: 2024-11-01
网络出版日期: 2024-12-16
基金资助
贵州省自然科学基金(黔科合基础-ZK[2023]重点048); 贵州省教育厅基金(黔教技[2023]088); 高层次人才科研启动基金(LPSSY-KYJJ201909); 六盘水师范学院科研培育项目(LPSSYLPY202330); 贵州省煤炭清洁利用重点实验室(黔科合平台-人才[2020]2001); 六盘水师范学院科技创新团队(LPSSYKJTD201904)
Oxalyl Monoamide Ligand-Promoted Palladium-Catalyzed Suzuki Coupling Reactions in Aqueous Media at Low Doses
Received date: 2024-11-01
Online published: 2024-12-16
Supported by
Natural Science Foundation of Guizhou Province (qiankehejichu-ZK[2023]zhongdian048); Foundation of Guizhou Educational Committee (qianjiaoji [2023]088); High-level Talents Research Start-up Fund(LPSSY-KYJJ201909); Liupanshui Normal University Scientific Research and Cultivation Projects(LPSSYLPY202330); Guizhou Provincial Key Laboratory of Coal Clean Utilization (qiankehepingtai-rencai [2020]2001); Science and Technology Innovation Team at Liupanshui Normal University(LPSSYKJTD201904)
杨雪 , 刘妍伶 , 陈霞 , 周晓玉 , 王爱玲 , 刘海龙 . 草酰单胺配体促进的低剂量钯催化水相Suzuki偶联反应[J]. 化学学报, 2025 , 83(4) : 354 -359 . DOI: 10.6023/A24110332
A practical and environmentally friendly low-dose palladium-catalyzed Suzuki coupling reaction system, utilizing simple oxalyl monoamides as ligands is introduced in this work. The reaction is conducted in a green solvent system composed of ethanol and water in a volume ratio of 1∶2, which not only reduces the environmental impact but also enhances the solubility of the reactants. Even at a low catalyst loading of n(S)/n(C)=70000, this system demonstrates excellent catalytic efficiency, yielding outstanding product yields of up to 98%. This is a substantial improvement over traditional methods that require higher catalyst concentrations. The study demonstrates the broad applicability of this reaction system to a variety of halogenated heteroaryl and aryl boronic acids, which are common substrates in the synthesis of complex molecules and pharmaceuticals. The high and consistent yields from the reactions validate the system's reliability and robustness. This work not only provides a more sustainable approach to Suzuki-Miyaura Cross-Coupling (SMCC) but also broadens the utility of oxalyl monoamide ligands in catalysis, paving the way for further exploration and application in the field of homogeneous catalysis. In summary, the development of this low-dose palladium-catalyzed Suzuki coupling system with oxalyl monoamides as ligands offers a green and efficient alternative for the synthesis of biaryl compounds. The system's versatility and the high yields obtained underscore its potential for broader applications in organic synthesis and catalysis research. This work contributes significantly to the ongoing efforts to develop more sustainable and efficient catalytic processes in chemical synthesis, making it a valuable addition to the field of green chemistry and catalysis.
| [1] | Farhang, M.; Akbarzadeh, A. R.; Rabbani, M.; Ghadiri, A. M. Polyhedron 2022, 227, 116124. |
| [2] | Hirota, Y.; Ando, S.; Ishizuka, T. Tetrahedron 2022, 108, 132668. |
| [3] | Isai Ortega-Gaxiola, J.; Valdés, H.; Rufino-Felipe, E.; Toscano, R. A.; Morales-Morales, D. Inorg. Chim. Acta 2020, 504, 119460. |
| [4] | Nandhini, R.; Vijayan, P.; Venkatachalam, G. J. Organomet. Chem. 2020, 907, 121080. |
| [5] | Nagalakshmi, V.; Sathya, M.; Premkumar, M.; Kaleeswaran, D.; Venkatachalam, G.; Balasubramani, K. J. Organomet. Chem. 2020, 914, 121220. |
| [6] | Zhang, Y.; Zhang, R.; Ni, C.; Zhang, X.; Li, Y.; Lu, Q.; Zhao, Y.; Han, F.; Zeng, Y.; Liu, G. Tetrahedron Lett. 2020, 61, 151541. |
| [7] | Saxena, P.; Murugavel, R. J. Organomet. Chem. 2018, 868, 76. |
| [8] | Thunga, S.; Poshala, S.; Anugu, N.; Konakanchi, R.; Vanaparthi, S.; Kokatla, H. P. Tetrahedron Lett. 2019, 60, 2046. |
| [9] | Czompa, A.; Pásztor, B. L.; Sahar, J. A.; Mucsi, Z.; Bogdán, D.; Ludányi, K.; Varga, Z.; Mándity, I. M. RSC Adv. 2019, 9, 37818. |
| [10] | Riadi, Y.; Lazar, S.; Guillaumet, G. C. R. Chimie 2019, 22, 294. |
| [11] | Nobre, S. M.; Cavalheiro, V. M. S.; Duarte, L. S. J. Mol. Struct. 2018, 1171, 594. |
| [12] | Louvis, A. D.; Silva, N. A. A.; Semaan, F. S.; Da Silva, F. D.; Saramago, G.; de Souza, L. C. S. V.; Ferreira, B. L. A.; Castro, H. C.; Salles, J. P.; Souza, A. L. A.; Faria, R. X.; Ferreira, V. F.; Martins, D. D. New J. Chem. 2016, 40, 7643. |
| [13] | Li, X.; Zhang, H.; Hu, Q.; Jiang, B.; Zeli, Y. Synlett. Commun. 2018, 48, 3123. |
| [14] | Sarkar, P.; Ahmed, A.; Ray, J. K. Tetrahedron Lett. 2020, 61, 151701. |
| [15] | Mai, S.; Li, W.; Li, X.; Zhao, Y.; Song, Q. Nat Commun 2019, 10, 5709. |
| [16] | Cartagenova, D.; Bachmann, S.; Püntener, K.; Scalone, M.; Newton, M. A.; Esteves, F. A. P.; Rohrbach, T.; Zimmermann, P. P.; van Bokhoven, J. A.; Ranocchiari, M. Catal. Sci. Technol. 2022, 12, 954. |
| [17] | Zhou, T.; Ji, C. L.; Hong, X.; Szostak, M. Chem. Sci. 2019, 10, 9865. |
| [18] | Alza, E.; Laraia, L.; Ibbeson, B. M.; Collins, S.; Galloway, W. R. J. D.; Stokes, J. E.; Venkitaraman, A. R.; Spring, D. R. Chem. Sci. 2015, 6, 390. |
| [19] | Miyaura, N.; Suzuki, A. Chem. Rev. 1995, 95, 2457. |
| [20] | Suzuki, A. Angew. Chem. Int. Ed. 2011, 30, 6722. |
| [21] | Surry, D. S.; Buchwald, S. L. Chem. Sci. 2011, 2, 27. |
| [22] | Shi, Y.; Derasp, J. S.; Maschmeyer, T.; Hein, J. E. Nat. Commun. 2024, 15, 5436. |
| [23] | Martin, R.; Buchwald, S. L. Acc. Chem. Res. 2008, 41, 1461. |
| [24] | Colacot, T. J. PMR 2011, 55, 84. |
| [25] | Kadu, B. S. Catal. Sci. Technol. 2021, 11, 1186. |
| [26] | Hooshmand, S. E.; Heidari, B.; Sedghi, R.; Varma, R. S. Green Chem. 2019, 21, 381. |
| [27] | Beletskaya, I. P.; Alonso, F.; Tyurin, V. Coord. Chem. Rev. 2019, 385, 137. |
| [28] | Li, G.; Lei, P.; Szostak, M.; Casals-Crua?as, E.; Poater, A.; Cavallo, L.; Nolan, S. P. ChemCatChem 2018, 10, 3096. |
| [29] | Melvin, P. R.; Nova, A.; Balcells, D.; Dai, W.; Hazari, N.; Hruszkewycz, D. P.; Shah, H. P.; Tudge, M. T. ACS Catal. 2015, 5, 3680. |
| [30] | Marion, N.; Navarro, O.; Mei, J.; Stevens, E. D.; Scott, N. M.; Nolan, S. P. J. Am. Chem. Soc. 2006, 128, 4101. |
| [31] | Fantasia, S.; Nolan, S. P. Chem. Eur. J. 2008, 14, 6987. |
| [32] | Dong, S.; Liu, X.; Feng, X. Acc. Chem. Res. 2022, 55, 415. |
| [33] | He, Y.; Zhu, S. Chin. J. Org. Chem. 2020, 40, 4377. (in Chinese) |
| [33] | (何玉立, 朱少林, 有机化学, 2020, 40, 4377.) |
| [34] | Xu, K.; Wang, Y.; Wang, X.; Hu, P.; Pan, B.; Zhang, W.; Chen, Z.; Zhou, Y.; Liu, X. Chin. J. Chem. 2024, 42, 1474. |
| [35] | Liu, H.; Liu, H.; Li, R.; Chen, H. Tetrahedron Lett. 2014, 55, 415. |
| [36] | Giesbrecht, G. R.; Shafir, A.; Arnold, J. Inorg. Chem. 2001, 40, 6069. |
| [37] | Braga, A. A. C.; Ujaque, G.; Maseras, F. Organometallics 2006, 25, 3647. |
| [38] | Sherwood, J.; Clark, J. H.; Fairlamb, I. J. S.; Slattery, J. M. Green Chem. 2019, 21, 2164. |
| [39] | Lipshutz, B. H.; Petersen, T. B.; Abela, A. R. Org. Lett. 2008, 10, 1333. |
| [40] | Nelson, C. B.; L’Heureux, S. J.; Wong, M. J.; Kuhn, S. L.; Ghiglietti, E.; Lipshutz, B. H. Green Chem. 2024, 26, 10115. |
/
| 〈 |
|
〉 |