

Ag掺杂对铁酸铋系化合物光催化性能的影响
收稿日期: 2024-12-04
网络出版日期: 2025-02-14
基金资助
内蒙古自治区高等学校青年科技人才项目(NJYT23002); 内蒙古工业大学科学研究项目基金(ZY201807)
Effect of Ag Doping on the Photocatalytic Performance of Bismuth Ferrite-based Compounds
Received date: 2024-12-04
Online published: 2025-02-14
Supported by
Youth Science and Technology Talents Support Project of Inner Mongolia Autonomous Region(NJYT23002); Science Project of Inner Mongolia University of Technology(ZY201807)
胡国伟 , 王晓欢 , 原志鹏 , 新巴雅尔 , 乌力吉贺希格 . Ag掺杂对铁酸铋系化合物光催化性能的影响[J]. 化学学报, 2025 , 83(3) : 229 -236 . DOI: 10.6023/A24120364
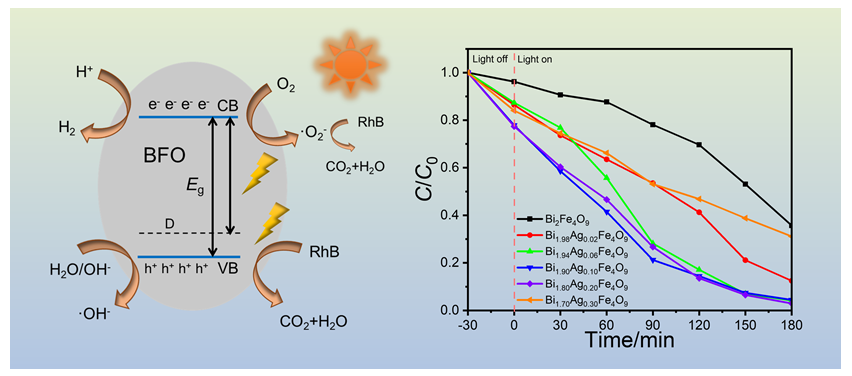
Bismuth ferrite-based compounds, such as BiFeO3 and Bi2Fe4O9, have attracted significant attention as photocatalytic materials due to their favorable structural properties and stability. In this study, Ag-doped BiFeO3 and Bi2Fe4O9 powders were synthesized using the sol-gel method, and their photocatalytic properties were evaluated through the degradation of Rhodamine B (RhB) under ultraviolet (UV) light. The results showed that Ag doping notably enhanced the photocatalytic properties of Bi2Fe4O9. Specifically, 3 mol% Ag-doped Bi2Fe4O9 exhibited an impressive RhB degradation rate of 96%, indicating its high efficiency for photocatalytic applications. In contrast, Ag doping in BiFeO3 led to the formation of mixed phases, including Bi2Fe4O9 and Bi25FeO40, and resulted in only a modest improvement in photocatalytic performance, with a RhB degradation rate of 50%. These findings highlight the superior photocatalytic activity of Bi2Fe4O9 compared to BiFeO3, emphasizing the importance of material composition and doping concentration in optimizing photocatalytic efficiency. Moreover, UV-Vis diffuse reflectance spectroscopy was used to investigate the electronic properties of the materials. The analysis revealed that Bi2Fe4O9 exhibits a dual-bandgap feature with values of 1.28 eV and 1.36 eV, which enhances the material's ability to absorb visible light and improves its photocatalytic performance under ambient light conditions. To explore the mechanisms behind these improvements, density functional theory (DFT) calculations were conducted. The results indicate that the Ag 4d orbitals introduce defect energy levels near the valence band maximum of Bi2Fe4O9, effectively narrowing the bandgap. This facilitates more efficient charge separation, which is crucial for enhancing photocatalytic properties. Fluorescence spectroscopy further confirmed that Ag doping effectively inhibits the recombination of photogenerated electron-hole pairs, further boosting the photocatalytic efficiency of Bi2Fe4O9. This study provides valuable insights into the potential of Ag-doped bismuth ferrite-based compounds for advanced photocatalysis, and pave the way for the development of efficient photocatalysts for environmental and energy-related applications, such as water purification and solar energy conversion.

Key words: Bismuth ferrite; photocatalysis; doping; first-principles; defect energy levels
| [1] | Xia, X. L.; Yang, X. Y.; Huang, W.; Xia, X. X.; Yan, D. Y. Nano-Micro Lett. 2022, 14, 33. |
| [2] | Sun. P. F.; Lin, M. C.; Chen, G. S.; Jiang, M. Sci. China Chem. 2016, 59, 1616. |
| [3] | Zhang, J. P.; Hu, Y.; Zhang, L. A.; Zhou, J. P.; Lu, A. Nano-Micro Lett. 2023, 15, 8. |
| [4] | Wang, B.; Tang, W.; Zhao, H.-X.; Long, L.-S; Zheng, L.-S. Acta Chim. Sinica 2021, 79, 119 (in Chinese). |
| [4] | (王宾, 唐雯, 赵海霞, 龙腊生, 郑兰荪, 化学学报, 2021, 79, 119.) |
| [5] | Xie, M.-S.; Wu, X.-X.; Wang, G.; Lin, L.-L; Feng, X.-M. Acta Chim. Sinica 2014, 72, 856 (in Chinese). |
| [5] | (谢明胜, 武晓霞, 王刚, 林丽丽, 冯小明, 化学学报, 2014, 72, 856.) |
| [6] | Zhang, C. Y.; Song, A.; Huang, Q. R.; Cao, Y. H.; Zhong, Z. Y.; Liang, Y. C.; Zhang, K.; Liu, C. C.; Huang, F.; Cao, Y. Nano-Micro Lett. 2023, 15, 140. |
| [7] | Zhou, L.; Shao, M. F.; Wei, M.; Duan, X. J. Energy Chem. 2017, 26, 1094. |
| [8] | Peng, K.; Fan, Z. Y.; Wang, Y. Q.; Xie, Y.; Ling, Y. Int. J. Hydrogen Energy 2024, 60, 835. |
| [9] | Reddy, P. A. K.; Reddy, P. V. L.; Kwon, E.; Kim, K. H.; Akter, T.; Kalagara, S. Environ. Int. 2016, 91, 94. |
| [10] | Saad, A. M.; Abukhadra, M. R.; Ahme, S. A. K.; Elzanaty, A. M.; Mady, A. H.; Betiha, M. A.; Shim, J. J.; Rabie, A. M. J. Environ. Manage. 2020, 258, 110043. |
| [11] | Hu, L. X.; Chen, F. Y.; Hu, P. F.; Zou, L. P.; Hu, X. J. Mol. Catal. A Chem. 2016, 411, 203. |
| [12] | Botta, S. G.; Nav??o, J. A.; Hidalgo, M. C.; Restrepo, G. M.; Litter, M. I. J. Photochem. Photobiol. A 1999, 129, 89. |
| [13] | Tavker, N.; Sharma, M. J. Environ. Manage. 2020, 255, 109906. |
| [14] | Das, S.; Jayaraman, V. Prog. Mater. Sci. 2014, 66, 112. |
| [15] | Zhang, J. j.; Qi, P.; Li, J.; Zheng, X. C.; Liu, P.; Guan, X. X.; Zheng, G. P. J. Ind. Eng. Chem. 2018, 61, 407. |
| [16] | Ran, J. R.; Yu, J. G.; Jaroniec, M. Green Chem. 2011, 13, 2708. |
| [17] | Tahmasebi, N.; Mirzavand, S.; Hakimyfard, A.; Barzegar, S. Adv. Powder Technol. 2018, 30, 257. |
| [18] | Patial, S.; Hasija, V.; Raizada, P.; Singh, P.; Singh, A. A. P. K.; Asiri, A. M. J. Environ. Chem. Eng. 2020, 8, 103791. |
| [19] | Malathi, A.; Madhanvan, J.; Ashokkumar, M.; Arunachalam, P. Appl. Catal. A-Gen. 2018, 555, 47. |
| [20] | Karamian, E.; Sharifnia, S. MSEB 2018, 238-239, 142. |
| [21] | Haroonabadi, L.; Sharifnia, S. Int. J. Hydrogen Energy 2024, 82, 1263. |
| [22] | Lee, J. H.; Lee, H.; Kang, M. Mater. Lett. 2016, 178, 316. |
| [23] | Song, Y. K.; Bao, Z. Q.; Ge, X. L.; Hu, S. H.; Meng, M. F.; Gu, Y. Y. Appl. Surf. Sci. 2024, 671, 160676. |
| [24] | Zhu, Q. Q.; Shi, X. Y.; Zhang, X. J.; Zhang, X.; Wang, Y. Z.; Wang, C. C. J. Alloys Compd. 2024, 997, 174887. |
| [25] | Liu, Y. J.; Xing, C. C.; Yao, Z. F.; Deng, Q. C.; Liang, T.; Zhang, S. M.; Pan, J. H.; Yu, Z. B.; Xie, T.; Li, R.; Hou, Y. P. J. Colloid Interface Sci. 2025, 677, 1095. |
| [26] | Fang, Y. Z.; Kong, X. J.; Wang, D. T.; Liu, J. H.; Cui, S. X. J. Phys. Chem. Solids 2019, 127, 107. |
| [27] | Cheng, B. J.; Li, X. Q.; Li, H. B.; Zhang, W. Z.; Han, R. Q.; Zhang, Y. M.; Xiang, J. J. Energy Storage. 2024, 79, 110137. |
| [28] | Wu, Z. Z.; Shi, J.; Deng, H. P. Sep. Purif. Technol. 2024, 349, 127779. |
| [29] | Zhang, C. M.; Wang, R. X.; Zhang, X. C.; Dong, Z. Y.; Wu, W. S.; Xue, J. B.; Fan, C. M. Sep. Purif. Technol. 2024, 342, 127087. |
| [30] | Lomanova, N. A.; Pleshakov, I. V.; Volkov, M. P.; Yastrebov, S. G.; Kenges, K.; Ugolkov, V. L.; Osipov, A. V.; Siyuan, T.; Buryanenko, I. V.; Semenov, V. G. Inorg. Chem. Commun. 2024, 161, 112109. |
| [31] | Qi, S. Y.; Guan, L.; Zhang, R. Y.; Wu, S. Q.; Zhang, K. Y. Opt. Mater. 2023, 142, 114112. |
| [32] | Wang, L. L.; Meng, S. A.; Chen, G. L.; Yang, X. D.; Yan, Y.; Chen, R. Micro Nano Lett. 2020, 15, 713. |
| [33] | Rao, K. S.; Kamath, S. M.; Kumar, R. R.; Kavitha, G.; MeherAbhinav, E.; Shri, S. S.; Induja, S.; Gopalakrishnan, C. Mater. Lett. 2021, 297, 129960. |
| [34] | Wang, G. M.; Yan, S. H.; Sun, J.; Wang, S. G.; Deng, Q. R. J. Mater. Sci.-Mater. El. 2017, 28, 4371. |
| [35] | Wang, K.; Xu, X. G.; Lu, L. Y.; Li, A.; Han, X. D.; Wu, Y.; Miao, J.; Jiang, Y. Chem. Phys. Lett. 2019, 715, 129. |
| [36] | Wang, G. M.; Cheng, D.; He, T. C.; Hu, Y. Y.; Deng, Q. R.; Mao, Y. W.; Wang, S. G. J. Mater. Sci. Mater. Electron. 2019, 30, 10923. |
| [37] | Li, S. J.; Hu, S. W.; Xu, K. B.; Jiang, W.; Hu, J. Q.; Liu, J. S. Mater. Lett. 2017, 188, 368. |
| [38] | Wang, Y. Y.; Yang, J. H.; Zhang, Z. X.; Zhao, P. J.; Chen, Y. Q.; Guo, Y.; Luo, X. G. Int. J. Biol. Macromol. 2024, 269, 131885. |
| [39] | Fan, T.; Chen, C. C.; Tang, Z. H. RSC Adv. 2016, 6, 9994. |
| [40] | Singh, A.; Singh, D.; Ahmed, B.; Ojha, A. K. Mater. Chem. Phys. 2022, 277, 125531. |
| [41] | Khalid, N. R.; Mazia, U.; Tahir, M. B.; Noaz, N. A.; Javid, M. A. J. Mol. Liq. 2020, 313, 113522. |
| [42] | Ersoz, E.; Yildirim, O. A. J. Korean Ceram. Soc. 2022, 59, 655. |
| [43] | Chen, Y.; Li, J.; Zhai, B. Y.; Liang, Y. N. Colloids Surf. A Physicochem. Eng. Asp. 2019, 568, 429. |
| [44] | Sundararajan, M.; Bonisha, B.; Ubaidullah, M.; Shaikh, S. M. F.; Revathi, S.; Thiripurasundari, D.; Dhiwahar, A. T.; Pandit, B.; Dash, C. S.; Shahazad, M. Mater. Res. Bull. 2022, 154, 111911. |
| [45] | Sultana, R.; Liba, S. I.; Rahman, M. A.; Yeachin, N.; Syed, I. M.; Bhuiyan, M. A. Surf. Interfaces 2023, 42 (Part A), 103302. |
| [46] | Phuruangrat, A.; Buapoon, S.; Bunluesak, T.; Suebsom, P.; Wannapop, S.; Thongtem, T.; Thongtem, S. Solid State Sci. 2022, 128, 106881. |
| [47] | Sundararajan, M.; Sailaja, V.; Kennedy, J. L.; Vijaya, J. J. Ceram. Int. 2017, 43, 540. |
| [48] | Zhang, L.; He, Y. M.; Wu, Y.; Wu, T. H. MSEB 2011, 176, 1497. |
| [49] | Zhang, Y. Y.; Guo, Y. P.; Duan, H. N.; Li, H.; Yang, L.; Wang, P.; Sun, C. Y.; Xu, B. Y.; Liu, H. Z. RSC Adv. 2014, 4, 28209. |
| [50] | Wang, T. T.; Deng, H. M.; Zhou, W. L.; Meng, X. K.; Yang, P. X.; Chu, J. H. Ceram. Int. 2017, 43, 6453. |
| [51] | Kresse, G.; Furthmüller, J. Comput. Mater. Sci. 1996, 6, 15. |
| [52] | Jiang, H.; Gomez-Abal, R. I.; Rinke, P.; Scheffler, M. Phys. Rev. B 2010, 82, 045108. |
| [53] | Wuliji, H. X. G.; Zhao, K. P.; Cai, X. M.; Jing, H. R.; Wang, Y. W.; Huang, H. R.; Wei, T. R.; Zhu, H.; Shi, X. Mater. Today Phys. 2023, 35, 101129. |
/
| 〈 |
|
〉 |