

收稿日期: 2025-01-02
网络出版日期: 2025-03-13
基金资助
甘肃省科技重大专项(22ZD6FA006); 及国家自然科学基金(21871123); 及国家自然科学基金(22171120)
C(sp3)—H Bond Oximinylation
Received date: 2025-01-02
Online published: 2025-03-13
Supported by
Science and Technology Major Program of Gansu Province of China(22ZD6FA006); National Natural Science Foundation of China(21871123); National Natural Science Foundation of China(22171120)
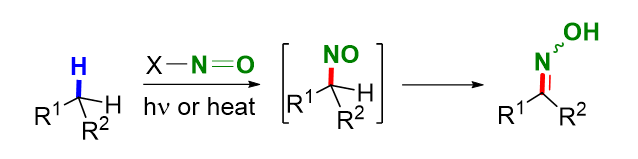
肟是一种常见有机化合物, 在化学、生物、环境、医药、农药、材料等领域及相关的工业生产中具有多种用途. 在众多合成方法中, 将烃类化合物碳氢键直接转化为肟官能团无疑是合成肟最直接和有效的手段. 活性亚甲基和甲基C(sp3)—H键的肟化反应通常依赖于亚硝酸、亚硝酰氯、亚硝酸盐和亚硝酰酯等肟化试剂, 其中亚硝酰阳离子(NO⁺)作为关键中间体参与反应. 相比之下, 自由基介导的肟化反应展现出更广泛的底物适用性, 甚至能够作用于仅含有未活化C(sp³)—H键的底物. 从反应机理来看, 该过程通常通过光照或加热引发亚硝基化合物的均裂, 生成一个以杂原子为中心的自由基和一氧化氮. 随后, 以杂原子为中心的自由基从底物中攫取一个氢原子, 形成烷基自由基. 该烷基自由基进一步参与后续的自由基反应, 并最终与一氧化氮发生偶联. 这种偶联反应生成亚硝基中间体, 随后通过异构化转化为目标肟产物. 梳理了从20世纪初至今饱和C(sp3)—H键的肟化反应, 按照反应历程不同, 分别对活泼亚甲基及甲基C(sp3)—H肟化反应、自由基介导的饱和C(sp3)—H键的肟化反应及金属介导的饱和C(sp3)—H键的肟化反应进行了综述, 并对该领域的局限性和未来发展方向做出了简要分析.
徐安佗 , 李骏一 , 刘强 . 饱和C(sp3)—H键肟化反应[J]. 化学学报, 2025 , 83(4) : 390 -400 . DOI: 10.6023/A25010005
Oxime is a widely used organic compound with numerous applications in chemistry, biology, environmental science, medicine, pesticides, materials, and related industrial production. Among the various synthetic methods, directly converting carbon-hydrogen bonds in hydrocarbon compounds into oximes is the most straightforward and practical approach for synthesizing oximes. The oximinylations of reactive methylene and methyl C(sp3)—H bonds often involve oximinylating reagents such as nitrous acid, nitrosyl halides, nitrite, and nitrosyl esters, with the nitrosyl cation as a crucial intermediate. In contrast, radical-mediated oximinylations have a broader substrate scope and can even be applied to substrates containing only unactivated C(sp3)—H bonds. Mechanistically, the reactions involve the homolytic cleavage of a nitroso compound via light irradiation or heating, generating a heteroatom-centered radical and a nitric oxide radical. The heteroatom-centered radical subsequently abstracts a hydrogen atom from the substrate to form an alkyl radical, which then undergoes subsequent radical reactions and finally couples with the nitric oxide radical. This coupling produces a nitroso intermediate, which isomerizes to oxime. This paper reviews the oximinylations of saturated C(sp3)—H bonds from the early 20th century to the present. It categorizes the reactions based on their mechanisms, including the oximinylations of reactive methylene and methyl C(sp3)—H bonds, radical-mediated oximinylations of C(sp3)—H bonds, and metal-mediated oximinylations of C(sp3)—H bonds. Additionally, this paper not only elucidates the development of the C(sp3)—H oximinylation reaction but also discusses the challenges in this field and offers future perspectives with the aim of providing insights for innovative applications in synthetic chemistry and industrial processes.

Key words: C—H bond activation; oxime; oximinylation; nitric oxide; nitrosyl cation
| [1] | Adams, J. P. J. Chem. Soc., 2000, 125. |
| [2] | Palacios, F.; de Retana, A. M. O.; de Marigorta, E. M.; de los Santos, J. M. Org. Prep. Proced. Int. 2002, 34, 219. |
| [3] | Kumar, R.; Chowdhury, B. Ind. Eng. Chem. Res. 2014, 53, 16587. |
| [4] | Bolotin, D. S.; Bokach, N. A.; Demakova, M. Y.; Kukushkin, V. Y. Chem. Rev. 2017, 117, 13039. |
| [5] | Bolotin, D. S.; Bokach, N. A.; Kukushkin, V. Y. Coord. Chem. Rev. 2016, 313, 62. |
| [6] | Ilinski, M.; Knorre, G. Ber. Dtsch. Chem. Ges. 1885, 18, 699. |
| [7] | Voloshin, Y. Z.; Novikov, V. V.; Nelyubina, Y. V. RSC Adv. 2015, 5, 72621. |
| [8] | Pan, T.-M.; Ye, J.-H.; Li, J.-N.; Gui, K.; Li, J.; Feng, J.-T.; Ma, Z.-Q.; Lei, P.; Gao, Y.-Q. J. Agric. Food. Chem. 2023, 71, 3164. |
| [9] | Ye, J.; Liu, X.; Zhou, R.; Hui, T.; Li, J.; Feng, J.; Ma, Z.-Q.; Gao, Y. Int. J. Food Microbiol. 2024, 409, 110461. |
| [10] | Balcerzak, L.; Surowiak, A. K.; Groborz, K.; Stró?ak, S.; Piekarska, K.; Strub, D. J. Toxicology 2023, 490, 153510. |
| [11] | Sekine, Y.JP 2004244408, 2004. |
| [12] | Komatsu, N.; Shimotomai, N.; Konosu, M. JP 62195309, 1987. |
| [13] | Oshchepkova, E. P.; Fridman, A. L.; Zalesov, V. S.; Kon'shina, L. O.; Kolobov, N. A.; Moiseev, I. K. SU 896140, 1982. |
| [14] | Tuloup, R.; Philippe, M.EP 1023894, 2000. |
| [15] | Chen, B.; Ning, Z.; Dong, Y. CN 112522718, 2021. |
| [16] | Cheng, Z.; Liu, Z.; Li, F.; Cao, W.; Zheng, Y.; Hu, Z. CN 110204272, 2019. |
| [17] | Worek, F.; Thiermann, H.; Wille, T. Chem. Biol. Interact. 2016, 259, 93. |
| [18] | Mercey, G.; Verdelet, T.; Renou, J.; Kliachyna, M.; Baati, R.; Nachon, F.; Jean, L.; Renard, P.-Y. Acc. Chem. Res. 2012, 45, 756. |
| [19] | Letendre, L.; Harriman, J.; Drag, M.; Mullins, A.; Malinski, T.; Rehbein, S. J. Vet. Pharmacol. Ther. 2017, 40, 35. |
| [20] | Nolan, T. J.; Lok, J. Curr. Pharm. Biotechnol. 2012, 13, 1078. |
| [21] | Bednarczyk-Cwynar, B.; Zaprutko, L. Phytochem. Rev. 2015, 14, 203. |
| [22] | Fylaktakidou, K. C.; Hadjipavlou-Litina, D. J.; Litinas, K. E.; Varella, E. A.; Nicolaides, D. N. Curr. Pharm. Des. 2008, 14, 1001. |
| [23] | Nikitjuka, A.; Jirgensons, A. Chem. Heterocycl. Compd. 2014, 49, 1544. |
| [24] | Lindell, S.; Dickhaut, J.; Jakobi, H.; Tiebes, J.; Jans, D.; Thoenessen, M.-T.; Waibel, J. M. EP 1236727, 2002. |
| [25] | Li, Q.; Cai, B.-G.; Li, L.; Xuan, J. Org. Lett. 2021, 23, 6951. |
| [26] | ābele, E.; Lukevics, E. J. H. Heterocycles 2000, 53, 2285. |
| [27] | Tan, Y.; Hartwig, J. F. J. Am. Chem. Soc. 2010, 132, 3676. |
| [28] | Lohse-Fraefel, N.; Carreira, E. M. Chem.-Eur. J. 2009, 15, 12065. |
| [29] | Ooi, T.; Takahashi, M.; Doda, K.; Maruoka, K. J. Am. Chem. Soc. 2002, 124, 7640. |
| [30] | Gagosz, F.; Zard, S. Z. Synlett 1999, 1999, 1978. |
| [31] | Nicastri, M. C.; Lehnherr, D.; Lam, Y.-H.; DiRocco, D. A.; Rovis, T. J. Am. Chem. Soc. 2020, 142, 987. |
| [32] | Benati, L.; Leardini, R.; Minozzi, M.; Nanni, D.; Scialpi, R.; Spagnolo, P.; Strazzari, S.; Zanardi, G. Angew. Chem., Int. Ed. 2004, 43, 3598. |
| [33] | Qi, X.-K.; Zheng, M.-J.; Yang, C.; Zhao, Y.; Guo, L.; Xia, W. J. Am. Chem. Soc. 2023, 145, 16630. |
| [34] | Zheng, Y.; Wang, Z.-J.; Ye, Z.-P.; Tang, K.; Xie, Z.-Z.; Xiao, J.-A.; Xiang, H.-Y.; Chen, K.; Chen, X.-Q.; Yang, H. Angew. Chem., Int. Ed. 2022, 61, e202212292. |
| [35] | Majhi, J.; Dhungana, R. K.; Rentería-Gómez, á.; Sharique, M.; Li, L.; Dong, W.; Gutierrez, O.; Molander, G. A. J. Am. Chem. Soc. 2022, 144, 15871. |
| [36] | Huang, T.; Liu, C.; Yuan, P.-F.; Wang, T.; Yang, B.; Ma, Y.; Liu, Q. Green Chem. 2024, 26, 9859. |
| [37] | Scheinbaum, M. L. J. Org. Chem. 1970, 35, 2785. |
| [38] | Prateeptongkum, S.; Jovel, I.; Jackstell, R.; Vogl, N.; Weckbecker, C.; Beller, M. Chem. Commun. 2009, 1990. |
| [39] | Yuan, P.-F.; Huang, T.; He, J.; Huang, X.-T.; Jin, X.-L.; Sun, C.-L.; Wu, L.-Z.; Liu, Q. Org. Chem. Front. 2021, 8, 5785. |
| [40] | Zheng, D.; Pl?ger, S.; Daniliuc, C. G.; Studer, A. Angew. Chem., Int. Ed. 2021, 60, 8547. |
| [41] | Wang, Z.; Wierich, N.; Zhang, J.; Daniliuc, C. G.; Studer, A. J. Am. Chem. Soc. 2023, 145, 8770. |
| [42] | Sang, J.-W.; Chen, H.; Zhang, Y.; Wang, J.; Zhang, W.-D. Green Chem. 2024, 26, 7849. |
| [43] | Yuan, P.-F.; Huang, X.-T.; Long, L.; Huang, T.; Sun, C.-L.; Yu, W.; Wu, L.-Z.; Chen, H.; Liu, Q. 2024, 63, e202317968. |
| [44] | Li, W.; Diao, C.-C.; Lu, Y.-L.; Li, H.-F. Org. Lett. 2024, 26, 6253. |
| [45] | Li, W.; Zhao, L.-T.; Diao, C.-C.; Zhang, G.-H.; Li, H.-F. Org. Lett. 2025, 27, 252. |
| [46] | Lan, J.-Y.; Li, X.-L.; Xu, M.-Y.; Zhang, B.; Luo, J.; Zhou, Y.; Wang, T. J. Org. Chem. 2025, 90, 250. |
| [47] | Yang, H.-X.; Li, M.-M.; Zhang, A.-J.; Guo, J.-F.; Yu, Y.-Q.; Ding, W. Chin. Chem. Lett. 2025, 36, 110425. |
| [48] | Chen, Z.-L.; Li, Q.-Q.; Studer, A.; Xuan, J. Sci. China: Chem. 2025, 68, 118. |
| [49] | Sang, J.-W.; Zhang, Y.; Xia, D.-D.; Hu, Z.-M.; Wang, J.-X.; Zhang, W.-D. Org. Chem. Front. 2025, 12, 869. |
| [50] | Aston, J. G.; Menard, D. F.; Mayberry, M. G. J. Am. Chem. Soc. 1932, 54, 1530. |
| [51] | Aston, J. G.; Mayberry, M. G. J. Am. Chem. Soc. 1935, 57, 1888. |
| [52] | Bennett, G. B.; Mason, R. B.; Alden, L. J.; Roach, J. B. Jr. J. Med. Chem. 1978, 21, 623. |
| [53] | Kataoka, M.; Ohno, M. Bull. Chem. Soc. Jpn. 1973, 46, 3474. |
| [54] | Taylor, E. C.; Dumas, D. J. J. Org. Chem. 1980, 45, 2485. |
| [55] | Rogic, M. M.; Vitrone, J.; Swerdloff, M. D. J. Am. Chem. Soc. 1977, 99, 1156. |
| [56] | Leis, J. R.; Pe?a, M. E.; Williams, D. L. H. J. Chem. Soc., Chem. Commun. 1987, 45. |
| [57] | Lee, S.; Fuchs, P. L. Can. J. Chem. 2006, 84, 1442. |
| [58] | Gao, X.; Zhang, F.; Deng, G.; Yang, L. Org. Lett. 2014, 16, 3664. |
| [59] | Tokuyama, H.; Cho, H.; Iwama, Y.; Noro, T.; Okano, K. Heterocycles 2014, 88, 1433. |
| [60] | Claisen, L.; Manasse, O. Ber. Dtsch. Chem. Ges. 1889, 22, 526. |
| [61] | Lynn, E. V. J. Am. Chem. Soc. 1919, 41, 368. |
| [62] | Lynn, E. V.; Arkley, H. L. J. Am. Chem. Soc. 1923, 45, 1045. |
| [63] | Mitchell, S.; Carson, S. J. Chem. Soc. 1936, 1005. |
| [64] | Naylor, M. A.; Anderson, A. W. J. Org. Chem. 1953, 18, 115. |
| [65] | Müller, E.; Metzger, H. Chem. Ber. 1954, 87, 1282. |
| [66] | Mackor, A.; Veenland, J. U.; de Boer, T. J. Recl. Trav. Chim. Pays-Bas 1969, 88, 1249. |
| [67] | Mackor, A.; de Boer, T. J. Recl. Trav. Chim. Pays-Bas 1970, 89, 151. |
| [68] | Mackor, A.; de Boer, T. J. Recl. Trav. Chim. Pays-Bas 1970, 89, 159. |
| [69] | Mackor, A.; de Boer, T. J. Recl. Trav. Chim. Pays-Bas 1970, 89, 164. |
| [70] | Müller, E.; B?ttcher, A. E. Tetrahedron Lett. 1970, 11, 3083. |
| [71] | Hirabayashi, T.; Sakaguchi, S.; Ishii, Y. Angew. Chem., Int. Ed. 2004, 43, 1120. |
| [72] | Hashimoto, M.; Sakaguchi, S.; Ishii, Y. Chem.-Asian J. 2006, 1, 712. |
| [73] | Wei, W.-T.; Zhu, W.-M.; Ying, W.-W.; Wu, Y.; Huang, Y.-L.; Liang, H. Org. Biomol. Chem. 2017, 15, 5254. |
| [74] | Wysocki, J.; Teles, J. H.; Dehn, R.; Trapp, O.; Sch?fer, B.; Schaub, T. ChemPhotoChem 2018, 2, 22. |
| [75] | Lebl, R.; Cantillo, D.; Kappe, C. O. React. Chem. Eng. 2019, 4, 738. |
| [76] | Griffiths, O. M.; Ruggeri, M.; Baxendale, I. R. Synlett 2020, 31, 1907. |
| [77] | Barton, D. H. R.; Hesse, R. H.; Pechet, M. M.; Smith, L. C. J. Chem. Soc., 1979, 1159. |
| [78] | Barton, D. H. R.; Beaton, J. M.; Geller, L. E.; Pechet, M. M. J. Am. Chem. Soc. 1961, 83, 4076. |
| [79] | Barton, D. H. R.; Lier, E. F.; McGhie, J. F. J. Chem. Soc. C 1968, 1031. |
| [80] | Suginome, H.; Kojima, T.; Orito, K.; Masamune, T. Tetrahedron 1971, 27, 291. |
| [81] | Corey, E. J.; Arnett, J. F.; Widiger, G. N. J. Am. Chem. Soc. 1975, 97, 430. |
| [82] | Ishmuratov, G. Y.; Kharisov, R. Y.; Shayakhmetova, A. K.; Botsman, L. P.; Shitikova, O. V.; Tolstikov, G. A. Chem. Nat. Compd. 2005, 41, 643. |
| [83] | Huang, T.; Yuan, P.-F.; Dong, K.; Zong, Y.-Y.; Liu, C.; Wang, R.-H.; Jin, X-L.; Liu, Q. Org. Chem. Front. 2023, 10, 4559. |
| [84] | Makarycheva-Mikhailova, A. V.; Gushchin, P. V.; Kopylovich, M. N.; Ganebnykh, I. N.; Charushin, V. N.; Haukka, M.; Pombeiro, A. J. L.; Kukushkin, V. Y. Inorg. Chem. Commun. 2006, 9, 869. |
| [85] | Yu, J.-T.; Lu, M. Org. Biomol. Chem. 2015, 13, 7397. |
/
| 〈 |
|
〉 |