

希托夫紫磷烯/SnS2范德华异质结作为直接全解水光催化剂的理论构建
收稿日期: 2024-12-27
网络出版日期: 2025-03-18
基金资助
辽宁省教育厅基本科研项目(LJKQZ20222272)
Theoretical Construction of Hittorf’s Violet Phosphorene/SnS2 van der Waals Heterojunction as Direct Photocatalyst for Overall Water Splitting
Received date: 2024-12-27
Online published: 2025-03-18
Supported by
Scientific Research Fund of the Education Department of Liaoning Province, China(LJKQZ20222272)
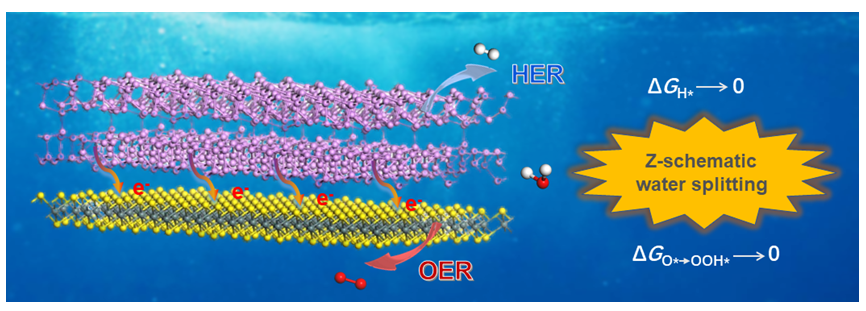
寻找高效的直接Z-型异质结构光催化剂分解水制氢, 被认为是解决能源危机和环境问题的有效途径之一. 本工作采用基于密度泛函理论(DFT)的第一性原理计算方法, 对构建的二维希托夫紫磷烯(HP)/SnS2异质结的电子结构、光学性质以及光催化性能开展了系统研究. 杂化泛函的计算结果表明, HP/SnS2异质结为直接带隙半导体, 其带隙值为1.30 eV. 交错的能带结构及其层间电荷转移诱导的内建电场诱发的直接Z-型载流子迁移机制, 使之具有很强的氧化还原能力引发光解水的反应. 光照下, HP侧的光生电子和SnS2侧的空穴提供的外电势能够大幅度降低HP/SnS2异质结析氢反应(HER)和析氧反应(OER)的吉布斯自由能, 从而使其具有远小于各自单层材料的吉布斯自由能, 表现出优异的析氢活性和析氧活性. 此外, 双轴应变可以有效调控HP/SnS2异质结的光催化能力以及光吸收特性. 在应变为–10%的时候, HP/SnS2异质结在可见光范围内的光吸收能力达到最强, 光吸收系数可高达1.71×106 cm-1. 通过比较水的氧化还原电位发现, 当应变在–10%~8%范围之间的时候, HP/SnS2异质结均可满足光催化全解水的条件, 其太阳能到氢能(Solar to Hydrogen, STH)转换效率最高可达到54.90%, 远超10%的商业要求. 综上所述, HP/SnS₂异质结是一种具有研究价值和应用潜力的全解水光催化剂材料.
卢一林 , 董盛杰 , 崔方超 , 薄婷婷 , 毛卓 . 希托夫紫磷烯/SnS2范德华异质结作为直接全解水光催化剂的理论构建[J]. 化学学报, 2025 , 83(4) : 377 -389 . DOI: 10.6023/A24120382
Seeking efficient direct Z-scheme heterojunction photocatalysts for water splitting and hydrogen production is an effective way to solve energy crises and environmental problems. Here, we used first-principles calculations based on density functional theory (DFT) to systematically study the electronic structure, optical properties, and photocatalytic performance of the constructed two-dimensional Hittorf’s violet phosphorene (HP)/SnS2 heterojunction. To achieve more accurate results, the Heyd-Scuseria-Ernzerhof (HSE06) calculations are performed to verify the results, especially for the calculations of the electronic structure and the optical properties. The hybrid functional computational results showed that the HP/SnS2 heterojunction is a direct bandgap semiconductor with a bandgap value of 1.30 eV. The staggered band structure and the built-in electric field induced by interlayer charge transfer result in a direct Z-scheme carrier migration mechanism, giving it a stronger oxidation-reduction ability to trigger water-splitting reactions. Under illumination conditions, the external voltage provided by the photogenerated electrons on the HP side and the photogenerated holes on the SnS2 side can significantly reduce the Gibbs free energy of hydrogen evolution reaction (HER) and oxygen evolution reaction (OER), which is substantially lower than Gibbs free energy required for single-layer HP and two-dimensional SnS2 to catalyze the same reaction, resulting in excellent hydrogen evolution activity and oxygen evolution activity. In addition, theoretical calculations showed that biaxial strain can effectively regulate the photocatalytic ability and light absorption characteristics of HP/SnS2 heterojunction. We found that at a strain of –10%, the HP/SnS2 heterojunction exhibits the strongest light absorption ability under visible light, with a light absorption coefficient of up to 1.71×106 cm-1. By comparing the oxidation-reduction potentials of water, it was found that HP/SnS2 heterojunction can meet the conditions for photocatalytic overall water splitting when the strain ranges from –10% to 8%. Its solar to hydrogen (STH) conversion efficiency can reach up to 54.90%, far exceeding the commercial requirement of 10%. In summary, HP/SnS2 heterojunction is an excellent candidate material for overall photocatalytic water splitting under visible light.

| [1] | Fujishima, A.; Honda, K. Nature 1972, 238, 37. |
| [2] | Zheng, Y.; Jiao, Y.; Zhu, Y. H.; Li, L. H.; Han, Y.; Chen, Y.; Du, A. J.; Jaroniec, M.; Qiao, S. Z. Nat. Commun. 2014, 5, 3783. |
| [3] | Voiry, D.; Shin, H. S.; Loh, K. P.; Chhowalla, M. Nat. Rev. Chem. 2018, 2, 0105. |
| [4] | Jin, H.; Guo, C.; Liu, X.; Liu, J.; Vasileff, A.; Jiao, Y.; Zheng, Y.; Qiao, S.-Z. Chem. Rev. 2018, 118, 6337. |
| [5] | Wu, Y.; Zhang, D.; Yin, H.; Chen, Z.; Zhao, W.; Chi, Y. Acta Chim. Sinica 2023, 81, 1148. (in Chinese) |
| [5] | (吴宇晗, 张栋栋, 尹宏宇, 陈正男, 赵文, 匙玉华, 化学学报, 2023, 81, 1148.) |
| [6] | Huang, G.; Li, K.; Luo, Y.; Zhang, Q.; Pan, Y.; Gao, H. Acta Chim. Sinica 2024, 82, 314. (in Chinese) |
| [6] | (黄广峥, 李坤玮, 罗艳楠, 张强, 潘远龙, 高洪林, 化学学报, 2024, 82, 314.) |
| [7] | Hou, F.; Liu, F.; Wu, H.; Qasim, M.; Chen, Y.; Duan, Y.; Feng, Z.; Liu, M. Chinese J. Chem. 2022, 41, 173. |
| [8] | Liu, J.; Li, C.; Liu, Y.; Wang, Y.; Fang, Q. Acta Chim. Sinica 2023, 81, 884. (in Chinese) |
| [8] | (刘建川, 李翠艳, 刘耀祖, 王钰杰, 方千荣, 化学学报, 2023, 81, 884.) |
| [9] | Wang, D.; Lan, Z.; Li, Y.; Huang, Y.; Yin, K.; Yang, L.; Bai, L.; Wei, D.; Yang, H.; Chen, H.; Luo, M. Chinese J. Chem. 2024, 43, 308. |
| [10] | Fong, C. Y.; Cohen, M. L. Phys. Rev. B 1972, 5, 3095. |
| [11] | Li, X.; Zhu, J.; Li, H. Appl. Catal. B 2012, 174, 123. |
| [12] | Wei, R.; Hu, J.; Zhou, T.; Zhou, X.; Liu, J.; Li, J. Acta Mater. 2014, 66, 163. |
| [13] | Guo, X.; Zhang, F.; Zhang, Y.; Hu, J. J. Mater. Chem. A 2023, 11, 7331. |
| [14] | Lin, J.; Liu, Y.; Liu, Y.; Huang, C.; Liu, W.; Mi, X.; Fan, D.; Fan, F.; Lu, H.; Chen, X. ChemSusChem 2019, 12, 961. |
| [15] | Sun, L.; Zhou, W.; Liu, Y.; Yu, D.; Liang, Y.; Wu, P. Appl. Surf. Sci. 2016, 389, 484. |
| [16] | Moniz, S. J. A.; Shevlin, S. A.; Martin, D. J.; Guo, Z.-X.; Tang, J. Energy Environ. Sci. 2015, 8, 731. |
| [17] | Marschall, R. Adv. Funct. Mater. 2014, 24, 2421. |
| [18] | Nasir, J. A.; Munir, A.; Ahmad, N. T. u.; Haq, Z.; Khan, Z.; Rehman, Z. Adv. Mater. 2021, 33, 2105195. |
| [19] | Liu, X.; Zhang, Q.; Ma, D. Solar RRL 2021, 5, 2000397. |
| [20] | Chen, X.; Han, W.; Yue, Q.; Zhang, Q.; Liang, Y.; Peng, C.; Yin, H. Inorg. Chem. 2023, 62, 17954. |
| [21] | Chen, C.; Shen, M.; Li, Y. Chem. Phys. 2021, 548, 111230. |
| [22] | Wang, J.; Luan, L.; Chen, J.; Zhang, Y.; Wei, X.; Fan, J.; Ni, L.; Liu, C.; Yang, Y.; Liu, J.; Tian, Y. Mater. Sci. Semicond. Process. 2023, 155, 107225. |
| [23] | Wang, B.; Wang, X.; Yuan, H.; Zhou, T.; Chang, J.; Chen, H. Int. J. Hydrogen Energy 2020, 45, 2785. |
| [24] | Zhang, L.; Huang, H.; Zhang, B.; Gu, M.; Zhao, D.; Zhao, X.; Li, L.; Zhou, J.; Wu, K.; Cheng, Y.; Zhang, J. Angew. Chem. Int. Ed. 2020, 59, 1074. |
| [25] | Schusteritsch, G.; Uhrin, M.; Pickard, C. J. Nano Lett. 2016, 16, 2975. |
| [26] | Ricciardulli, A. G.; Wang, Y.; Yang, S.; Samorì, P. J. Am. Chem. Soc. 2022, 144, 3660. |
| [27] | Zhang, B.; Wang, Z.; Huang, H.; Zhang, L.; Gu, M.; Cheng, Y.; Wu, K.; Zhou, J.; Zhang, J. J. Mater. Chem. A 2020, 8, 8586. |
| [28] | Lu, Y. L.; Dong, S.; Zhou, W.; Dai, S.; Zhou, B.; Zhao, H.; Wu, P. Phys. Chem. Chem. Phys. 2018, 20, 11967. |
| [29] | Lu, Y.-L.; Dong, S. J.; Li, J. S.; Mao, Z.; Wu, Y. Q.; Yang, L.-L. J. Phys. Chem. Solids 2022, 169, 110863. |
| [30] | Wang, X.; Ma, M.; Zhao, X.; Jiang, P.; Wang, Y.; Wang, J.; Zhang, J.; Zhang, F. Small Structures 2023, 4, 2300123. |
| [31] | Li, H.; Zhang, L.; Li, R.; Du, W.; Wu, B.; Lei, W.; Yu, J.; Liu, G.; Tan, T.; Zheng, L.; Liu, X. Nano Today 2023, 51, 101885. |
| [32] | Zhao, X.; Gu, M.; Zhai, R.; Zhang, Y.; Jin, M.; Wang, Y.; Li, J.; Cheng, Y.; Xiao, B.; Zhang, J. Small 2023, 19, 2302859. |
| [33] | Li, H.; Li, R.; Jing, Y.; Liu, B.; Xu, Q.; Tan, T.; Liu, G.; Zheng, L.; Wu, L. Z. ACS Catal. 2024, 14, 7308. |
| [34] | Wang, X.; Wang, Y.; Ma, M.; Zhao, X.; Zhang, J.; Zhang, F. Small 2024, 20, 2311841. |
| [35] | Lu, Y.-L.; Dong, S.; Cui, F.; Zhang, K.; Liu, C.; Li, J.; Mao, Z. Int. J. Hydrogen Energy 2025, 101, 222. |
| [36] | Lu, Y. L.; Dong, S.; He, H.; Li, J.; Wang, X.; Zhao, H.; Wu, P. Comput. Mater. Sci. 2019, 163, 209. |
| [37] | Sun, Y.; Cheng, H.; Gao, S.; Sun, Z.; Liu, Q.; Liu, Q.; Lei, F.; Yao, T.; He, J.; Wei, S.; Xie, Y. Angew. Chem. Int. Ed. 2012, 51, 8727. |
| [38] | Liu, J.; Hua, E. J. Phys. Chem. C 2017, 121, 25827. |
| [39] | Sun, Y.; Cheng, H.; Gao, S.; Sun, Z.; Liu, Q.; Liu, Q.; Lei, F.; Yao, T.; He, J.; Wei, S.; Xie, Y. Angew. Chem. Int. Ed. 2012, 51, 8727. |
| [40] | Zhang, R.; Sun, F. W.; Zhang, Z. H.; Liu, J.; Tian, Y.; Zhang, Y.; Wei, X.; Guo, T. T.; Fan, J. B.; Ni, L.; Duan, L. Appl. Surf. Sci. 2021, 535, 147825. |
| [41] | Wang, Y. L.; Tian, Y.; Lang, Z. L.; Guan, W.; Yan, L. K. J. Mater. Chem. A 2018, 6, 21056. |
| [42] | Zhang, Z.; Xie, Z.; Liu, J.; Tian, Y.; Zhang, Y.; Wei, X.; Duan, L. Phys. Chem. Chem. Phys. 2020, 22, 5873. |
| [43] | Zhang, Y.; Qiang, Z. B.; Ding, J. X.; Xie, K. X.; Duan, L.; Ni, L. CrystEngComm 2024, 26, 2621. |
| [44] | Wang, F.; Yang, C. L.; Wang, M. S.; Ma, X. J. Power Sources 2022, 532, 231352. |
| [45] | Garg, P.; Rawat, K. S.; Bhattacharyya, G.; Kumar, S.; Pathak, B. ACS Appl. Nano Mater. 2019, 2, 4238. |
| [46] | Peng, B.; Xu, L.; Zeng, J.; Qi, X.; Yang, Y.; Ma, Z.; Huang, X.; Wang, L. L.; Shuai, C. Catal. Sci. Technol. 2021, 11, 3059. |
| [47] | Bouziani, I.; Essaoudi, I.; Ahuja, R.; Ainane, A. Int. J. Hydrogen Energy 2023, 48, 35542. |
| [48] | He, C.; Han, F.; Zhang, W. Chin. Chem. Lett. 2022, 33, 404. |
| [49] | Zhang, W. X.; Yin, Y.; He, C. J. Phys. Chem. Lett. 2021, 12, 5064. |
| [50] | Dai, Z. N.; Sheng, W.; Xu, Y. J. At. Mol. Phys. 2024, 41, 061001. (in Chinese) |
| [50] | (戴卓旎, 盛威, 许英, 原子与分子物理学报, 2024, 41, 061001.) |
| [51] | Wang, J.; Chang, K.; Sun, Z.; Lee, J. H.; Tackett, B. M.; Zhang, C.; Chen, J. G.; Liu, C. J. Appl. Catal. B 2019, 251, 162. |
| [52] | Xu, K.; Huang, X.; Yang, Z. H. J. At. Mol. Phys. 2025, 42, 061002. (in Chinese) |
| [52] | (许康, 黄欣, 杨志红, 原子与分子物理学报, 2025, 42, 061002.) |
| [53] | Xia, F.; Yang, F. Energ. Fuel. 2022, 36, 4992. |
| [54] | Wirth, J.; Neumann, R.; Antonietti, M.; Saalfrank, P. Phys. Chem. Chem. Phys. 2014, 16, 15917. |
| [55] | Zhai, P.; Xia, M.; Wu, Y.; Zhang, G.; Gao, J.; Zhang, B.; Cao, S.; Zhang, Y.; Li, Z.; Fan, Z.; Wang, C.; Zhang, X.; Miller, J. T.; Sun, L.; Hou, J. Nat. Commun. 2021, 12, 4587. |
| [56] | Zhang, X.; Chen, A.; Zhang, Z.; Jiao, M.; Zhou, Z. J. Mater. Chem. A 2018, 6, 11446. |
| [57] | Zhao, Y.; Ma, D.; Zhang, J.; Lu, Z.; Wang, Y. Phys. Chem. Chem. Phys. 2019, 21, 20432. |
| [58] | Yuan, J.; Wang, C.; Liu, Y.; Wu, P.; Zhou, W. J. Phys. Chem. C 2018, 123, 526. |
| [59] | Zhou, S.; Yang, X.; Pei, W.; Liu, N.; Zhao, J. Nanoscale 2018, 10, 10876. |
| [60] | Pei, W.; Zhou, S.; Bai, Y.; Zhao, J. Carbon 2018, 133, 260. |
| [61] | Fu, C. F.; Sun, J.; Luo, Q.; Li, X.; Hu, W.; Yang, J. Nano Lett. 2018, 18, 6312. |
| [62] | Liang, K.; Wang, J.; Wei, X.; Zhang, Y.; Yang, Y.; Liu, J.; Duan, L. Physica E 2024, 155, 115825. |
| [63] | Xu, L.; Yang, Y.; Xin, C.; Jin, Z.; Chao, Y.; Wu, C.; Luo, K. W.; Wang, L. L.; Chen, T. Mater. Today Commun. 2024, 40, 109667. |
| [64] | Chen, X.; Han, W.; Jia, M.; Ren, F.; Peng, C.; Gu, Q.; Wang, B.; Yin, H. J. Phys. D: Appl. Phys. 2022, 55, 215502. |
| [65] | Chen, X.; Han, W.; Tian, Z.; Yue, Q.; Peng, C.; Wang, C.; Wang, B.; Yin, H.; Gu, Q. J. Phys. Chem. C 2023, 127, 6347. |
| [66] | Kresse, G.; Hafner, J. Phys. Rev. B 1993, 47, R558. |
| [67] | Kresse, G.; Hafner, J. Phys. Rev. B 1994, 49, 14251. |
| [68] | Blochl, P. E. Phys. Rev. B 1994, 50, 17953. |
| [69] | Perdew, J. P.; Burke, K.; Ernzerhof, M. Phys. Rev. Lett. 1996, 77, 3865. |
| [70] | Monkhorst, H. J.; Pack, J. D. Phys. Rev. B 1976, 13, 5188. |
| [71] | Grimme, S. J. Comput. Chem. 2006, 27, 1787. |
| [72] | Heyd, J.; Scuseria, G. E.; Ernzerhof, M. J. Chem. Phys. 2003, 118, 8207. |
| [73] | Martyna, G. J.; Klein, M. L.; Tuckerman, M. J. Chem. Phys. 1992, 97, 2635. |
/
| 〈 |
|
〉 |