

复相氧化钛晶相比例对Pt-WOx/TiO2催化剂结构及甘油氢解性能的影响
收稿日期: 2025-01-13
网络出版日期: 2025-03-25
基金资助
国家自然科学基金(22272030); 上海市重点实验室专项经费(2024DZSYS02)
Effect of Crystal Phase Ratio of Bi-Phase Titania on Structure and Catalytic Performance of Pt-WOx/TiO2 Catalyst in Glycerol Hydrogenolysis
Received date: 2025-01-13
Online published: 2025-03-25
Supported by
National Natural Science Foundation of China(22272030); Science and Technology Commission of Shanghai Municipality(2024DZSYS02)
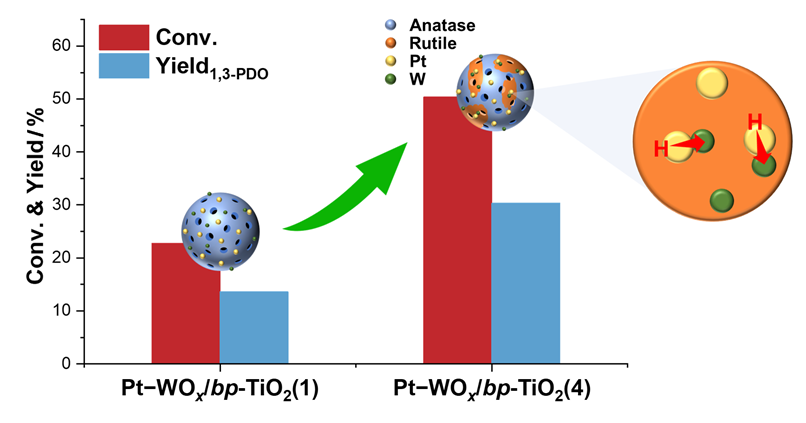
随着生物柴油工业的快速发展, 将副产物甘油选择性氢解为1,3-丙二醇(1,3-PDO)已成为重要的研究课题. 然而, 目前关于复相载体在甘油氢解催化剂中的应用及其对催化性能的影响的研究未见报道. 本研究通过配位介导自组装法合成了具有不同金红石/锐钛矿相比例的复相TiO2材料(bp-TiO2), 并以其为载体制备了Pt-WOx/bp-TiO2双功能催化剂, 旨在探讨TiO2载体晶相组成对甘油选择氢解性能的影响. 采用X射线衍射(XRD)、拉曼光谱(Raman)、N2物理吸附、CO脉冲吸附、透射电子显微镜(TEM)、吡啶吸附-傅里叶变换红外光谱(Py-IR)、程序升温氨脱附(NH3-TPD)和H2化学吸附等多种技术, 系统地表征了催化剂的物相组成、形貌、表面价态及组成、酸性和氢溢流能力等性质. 甘油氢解反应结果显示, 随着载体中金红石相含量的增加, Pt-WOx/bp-TiO2催化剂上的甘油转化率总体呈上升趋势, 而1,3-PDO的选择性保持在60%左右. 当载体中金红石相含量达到最高值29%时, 1,3-PDO的得率达到最高, 为30.3%. 该催化剂同时显示了良好的稳定性. TEM和X射线光电子能谱(XPS)表征表明, 相较于锐钛矿相, Pt和WOx物种更倾向于分布在金红石相表面上. 这种选择性分布有利于增加Pt-WOx接触面积, 从而增强了催化剂的氢溢流能力, 显著提升了目标产物1,3-PDO的得率. 本工作揭示了复相TiO2晶相组成对负载型Pt-WOx催化剂在甘油选择氢解反应中的重要影响, 为研发高性能负载催化剂提供了新的思路.
陈建华 , 姜兰 , 曾杨 , 谢颂海 , 裴燕 , 乔明华 . 复相氧化钛晶相比例对Pt-WOx/TiO2催化剂结构及甘油氢解性能的影响[J]. 化学学报, 2025 , 83(4) : 332 -340 . DOI: 10.6023/A25010018
Driven by advances in the biodiesel industry, converting glycerol, the major byproduct, into valuable chemicals such as 1,3-propanediol (1,3-PDO) via selective hydrogenolysis has emerged as an important research topic. However, there is a dearth in the study of the effect of the bi-phase support on the catalytic performance of the Pt-WOx-based catalyst in glycerol hydrogenolysis. In this contribution, we synthesized bi-phase TiO2 (bp-TiO2) materials with varying rutile to anatase phase ratios using a coordination-mediated self-assembly method by adjusting the amount of HCl added. These materials were then used as supports for Pt-WOx catalysts to investigate the effect of crystal phase composition of the support on glycerol hydrogenolysis to 1,3-PDO. The Pt-WOx/bp-TiO2 catalysts were systematically characterized by techniques including X-ray diffraction (XRD), Raman spectrometry, N2 physisorption, CO pulsed adsorption, transmission electron microscopy (TEM), pyridine adsorption-Fourier transform infrared spectrometry (Py-IR), temperature-programmed desorption of NH3 (NH3-TPD), and H2 chemisorption, focusing on the phase composition, distribution and dispersion of the Pt and WOx species, acidic property, and ability of hydrogen spillover. The XRD results revealed that with the increase in the HCl dosage, the content of the rutile phase in the support increased first, and then decreased, reaching a maximum of 29%. The TEM and X-ray photoelectron spectroscopy (XPS) results disclosed that the Pt particles and WOx species were inclined to distribute on the rutile phase. The H2 chemisorption results demonstrated that the introduction of the rutile phase greatly enhanced the hydrogen spillover ability of the catalysts. In glycerol hydrogenolysis, it is identified that the glycerol conversion generally improved with the increase in the content of the rutile phase in the support, while the selectivity to 1,3-PDO remained virtually constant at around 60%. Over the Pt-WOx/bp-TiO2(4) catalyst with the highest rutile content of 29%, the yield of 1,3-PDO reached the highest value of 30.3%. This catalyst also displayed good stability. It is plausible that the preferential distribution of the Pt and WOx species on the rutile surface is conducive to the formation of more Pt-WOx interfaces, which greatly enhances hydrogen spillover and thus boosts the yield of 1,3-PDO. This work elucidates the important role of the phase composition of bi-phase TiO2 support on the catalytic performance of the Pt-WOx-based catalyst in glycerol selective hydrogenolysis, which opens up new avenue for the design of high-performance glycerol hydrogenolysis catalysts by means of engineering the phase composition of the support.

| [1] | Hoekman, S. K.; Broch, A.; Robbins, C.; Ceniceros, E.; Natarajan, M. Renewable Sustainable Energy Rev. 2012, 16, 143. |
| [2] | Attarbachi, T.; Kingsley, M. D.; Spallina, V. Fuel 2023, 340, 127485. |
| [3] | Kaur, G.; Srivastava, A. K.; Chand, S. Biochem. Eng. J. 2012, 64, 106. |
| [4] | Zhu, F.; Liu, D.; Chen, Z. Green Chem. 2022, 24, 1390. |
| [5] | Shrirame, B. S.; Varma, A. R.; Sahoo, S. S.; Gayen, K.; Maity, S. K. Biomass Bioenergy 2023, 177, 106943. |
| [6] | Bhowmik, S.; Darbha, S. Catal. Rev. 2021, 63, 639. |
| [7] | Yang, M.; Jiao, Y.; Ren, Y. Prog. Chem. 2024, 36, 256. |
| [8] | Wu, F.; Jiang, H.; Zhu, X.; Lu, R.; Shi, L.; Lu, F. ChemSusChem 2021, 14, 569. |
| [9] | Fan, Y.; Cheng, S.; Wang, H.; Tian, J.; Xie, S.; Pei, Y.; Qiao, M.; Zong, B. Appl. Catal., B 2017, 217, 331. |
| [10] | Zeng, Y.; Jiang, L.; Zhang, X.; Xie, S.; Pei, Y.; Zhou, G.; Hua, W.; Qiao, M.; Li, Z.; Zong, B. ACS Sustainable Chem. Eng. 2022, 10, 9532. |
| [11] | Wang, Y.; Zhou, Z.; Wang, C.; Zhao, L.; Xia, Q. Front. Chem. 2022, 10, 1004925. |
| [12] | Zhou, G.; Dou, R.; Bi, H.; Xie, S.; Pei, Y.; Fan, K.; Qiao, M.; Sun, B.; Zong, B. J. Catal. 2015, 332, 119. |
| [13] | Feng, Y.; Zhang, Y.; Wang, J.; Ling, L.; Zhang, R.; Fan, M.; Hou, B.; Li, D.; Wang, B. ACS Catal. 2024, 14, 1874. |
| [14] | Zhang, W.; He, H.; Tian, Y.; Lan, K.; Liu, Q.; Wang, C.; Liu, Y.; Elzatahry, A.; Che, R.; Li, W.; Zhao, D. Chem. Sci. 2019, 10, 1664. |
| [15] | Zhang, H.; Banfield, J. F. J. Phys. Chem. B 2000, 104, 3481. |
| [16] | Ohsaka, T.; Izumi, F.; Fujiki, Y. J. Raman Spectrosc. 1978, 7, 321. |
| [17] | Deo, G.; Turek, A. M.; Wachs, I. E.; Machej, T.; Haber, J.; Das, N.; Eckert, H.; Hirt, A. M. Appl. Catal. A 1992, 91, 27. |
| [18] | Yan, J.; Wu, G.; Guan, N.; Li, L.; Li, Z.; Cao, X. Phys. Chem. Chem. Phys. 2013, 15, 10978. |
| [19] | Kim, D. S.; Ostromecki, M.; Wachs, I. E. J. Mol. Catal. A 1996, 106, 93. |
| [20] | Arribas, M. A.; Márquez, F.; Martínez, A. J. Catal. 2000, 190, 309. |
| [21] | Komornicki, S.; Radecka, M.; Sobas, P. J. Mater. Sci.: Mater. Electron. 2004, 15, 527. |
| [22] | Kim, T. Y.; Park, D. S.; Choi, Y.; Baek, J.; Park, J. R.; Yi, J. J. Mater. Chem. 2012, 22, 10021. |
| [23] | Ou, G.; Xu, Y.; Wen, B.; Lin, R.; Ge, B.; Tang, Y.; Liang, Y.; Yang, C.; Huang, K.; Zu, D.; Yu, R.; Chen, W.; Li, J.; Wu, H.; Liu, L.-M.; Li, Y. Nat. Commun. 2018, 9, 1302. |
| [24] | He, J.; Burt, S. P.; Ball, M.; Zhao, D.; Hermans, I.; Dumesic, J. A.; Huber, G. W. ACS Catal. 2018, 8, 1427. |
| [25] | Zhu, S.; Qiu, Y.; Zhu, Y.; Hao, S.; Zheng, H.; Li, Y. Catal. Today 2013, 212, 120. |
| [26] | García-Fernández, S.; Gandarias, I.; Requies, J.; Güemez, M. B.; Bennici, S.; Auroux, A.; Arias, P. L. J. Catal. 2015, 323, 65. |
| [27] | Lauriol-Garbey, P.; Postole, G.; Loridant, S.; Auroux, A.; Belliere-Baca, V.; Rey, P.; Millet, J. M. M. Appl. Catal. B 2011, 106, 94. |
| [28] | Jiang, F.; Zeng, L.; Li, S.; Liu, G.; Wang, S.; Gong, J. ACS Catal. 2014, 5, 438. |
| [29] | Gong, L.; Lu, Y.; Ding, Y.; Lin, R.; Li, J.; Dong, W.; Wang, T.; Chen, W. Appl. Catal. A 2010, 390, 119. |
| [30] | Deng, C.; Duan, X.; Zhou, J.; Chen, D.; Zhou, X.; Yuan, W. Catal. Today 2014, 234, 208. |
| [31] | Zhao, B.; Liang, Y.; Liu, L.; He, Q.; Dong, J. Green Chem. 2020, 22, 8254. |
| [32] | Wen, Y.; Shen, W.; Li, Y.; Fang, Y. React. Kinet., Mech. Catal. 2021, 132, 219. |
| [33] | Chen, J.; Huang, H.; Pan, H.; Cao, Y.; Jiang, L.; Xia, Q.; Meng, X.; Qiu, J.; Zuilhof, H.; Liu, S. ACS Sustainable Chem. Eng. 2024, 12, 6242. |
| [34] | Cheng, S.; Zeng, Y.; Pei, Y.; Fan, K.; Qiao, M.; Zong, B. Acta Chim. Sinica 2019, 77, 1054. (in Chinese) |
| [34] | (成诗婕, 曾杨, 裴燕, 范康年, 乔明华, 宗保宁, 化学学报, 2019, 77, 1054.) |
| [35] | Zeng, Y.; Jiang, L.; Zhang, X.; Xie, S.; Pei, Y.; Qiao, M.; Li, Z.; Xu, H.; Fan, K.; Zong, B. Acta Chim. Sinica 2022, 80, 903. (in Chinese) |
| [35] | (曾杨, 姜兰, 张晓昕, 谢颂海, 裴燕, 乔明华, 李振华, 徐华龙, 范康年, 宗保宁, 化学学报, 2022, 80, 903.) |
| [36] | Jiang, L.; Fan, Y.; Zhang, X.; Pei, Y.; Yan, S.; Qiao, M.; Fan, K.; Zong, B. Acta Chim. Sinica 2023, 81, 231. (in Chinese) |
| [36] | (姜兰, 范义秋, 张晓昕, 裴燕, 闫世润, 乔明华, 范康年, 宗保宁, 化学学报, 2023, 81, 231.) |
| [37] | Wang, L.; Stuckert, A. N.; Chen, H.; Yang, R. T. J. Phys. Chem. C 2011, 115, 4793. |
| [38] | Pevzner, S.; Pri-Bar, I.; Lutzky, I.; Ben-Yehuda, E.; Ruse, E.; Regev, O. J. Phys. Chem. C 2014, 118, 27164. |
| [39] | Fan, Y.; Cheng, S.; Wang, H.; Ye, D.; Xie, S.; Pei, Y.; Hu, H.; Hua, W.; Li, Z. H.; Qiao, M.; Zong, B. Green Chem. 2017, 19, 2174. |
| [40] | Lei, N.; Zhao, X.; Hou, B.; Yang, M.; Zhou, M.; Liu, F.; Wang, A.; Zhang, T. ChemCatChem 2019, 11, 3903. |
| [41] | Zhou, W.; Zhao, Y.; Wang, Y.; Wang, S.; Ma, X. ChemCatChem 2016, 8, 3663. |
| [42] | Conner, W. C., Jr.; Falconer, J. L. Chem. Rev. 1995, 95, 759. |
/
| 〈 |
|
〉 |