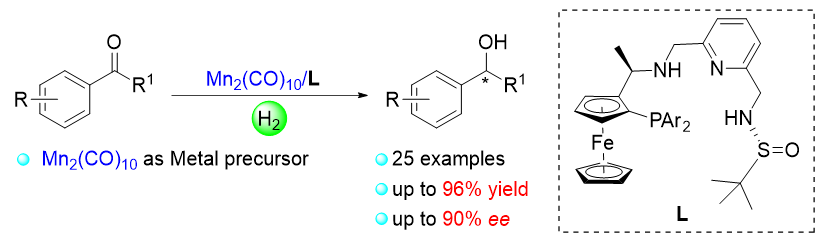
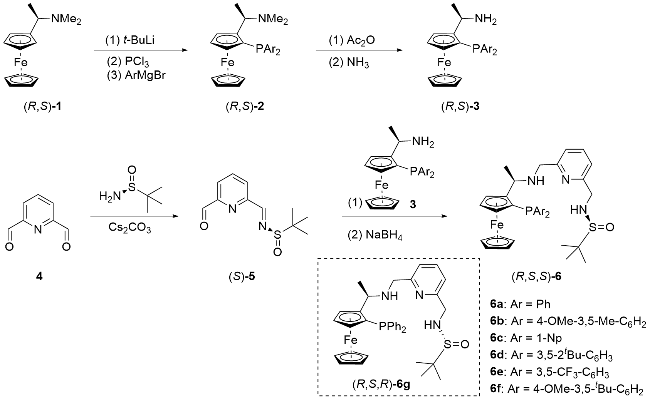
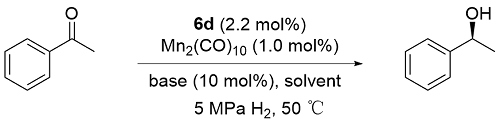
1 引言
2 结果与讨论
2.1 新型二茂铁配体的制备
表1 不对称氢化反应的金属和配体筛选Table 1 Metal precursor and ligand screening for asymmetric hydrogenation reactions |
| Entry | 配体 | 金属前体 | 转化率a/% | 对映选择性eeb/% |
|---|---|---|---|---|
| 1 | 6a | [Ir(COD)Cl]2 | 99 | 37 |
| 2 | 6g | [Ir(COD)Cl]2 | 65 | 14 |
| 3 | 6a | Mn(CO)5Br | 99 | 69 |
| 4 | 6a | Mn2(CO)10 | 99 | 67 |
| 5 | 6b | Mn2(CO)10 | 99 | 59 |
| 6 | 6c | Mn2(CO)10 | — | — |
| 7 | 6d | Mn2(CO)10 | 99 | 77 |
| 8 | 6e | Mn2(CO)10 | 47 | 79 |
| 9 | 6f | Mn2(CO)10 | 99 | 67 |
| 10 | 6g | Mn2(CO)10 | 99 | 56 |
a The conversion rate was determined by 1H NMR. b The enantioselectivity was determined by HPLC. |
2.2 不对称氢化反应条件的优化
表2 不对称氢化反应条件优化Table 2 Optimization of conditions for asymmetric hydrogenation |
| Entry | 溶剂 | 碱 | 转化率a/% | 对映选择性eeb/% |
|---|---|---|---|---|
| 1 | MeOH | KOtBu | 99 | 75 |
| 2 | iPrOH | KOtBu | 58 | 70 |
| 3 | EtOH | KOtBu | 99 | 77 |
| 4 | TFE | KOtBu | 18 | 68 |
| 5 | THF | KOtBu | NR | — |
| 6 | HFIP | KOtBu | NR | — |
| 7 | MTBE | KOtBu | NR | — |
| 8 | EtOH | NaOtBu | 99 | 76 |
| 9 | EtOH | LiOtBu | 99 | 78 |
| 10 | EtOH | Na2CO3 | 58 | 83 |
| 11 | EtOH | K2CO3 | 62 | 81 |
| 12 | EtOH | Cs2CO3 | 95 | 77 |
| 13 | EtOH | NaOMe | 98 | 77 |
| 14 | EtOH | LiOMe | 78 | 78 |
| 15 | EtOH | NaOH | 98 | 78 |
| 16 | EtOH | KOH | 99 | 78 |
| 17c | EtOH | KOtBu | 99 | 78 |
| 18d | EtOH | KOtBu | 99 | 84 |
a The conversion rate was determined by 1H NMR. b The enantioselectivity was determined by HPLC. c nS/nC=100/1, 3.5 MPa H2, 35 ℃. d nS/nC=100/1, 3.5 MPa H2, 35 ℃, 10 mol% HFIP. |