1 引言
2 结果与讨论
2.1 结构与形貌表征
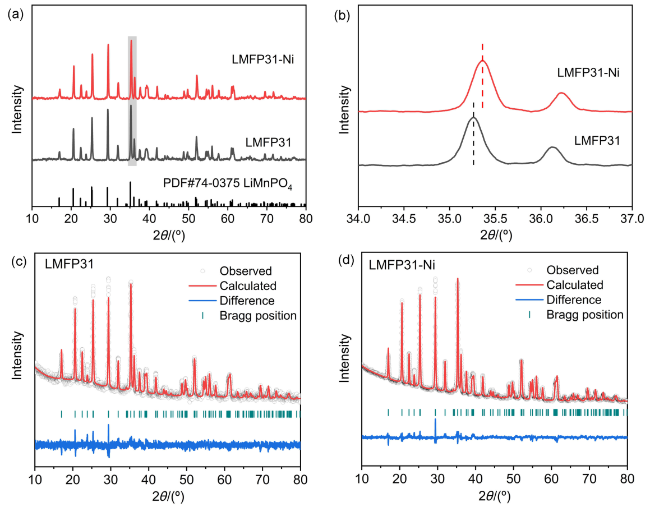
图1 (a) LMFP31和LMFP31-Ni的XRD图谱; (b) LMFP31和LMFP31-Ni在34°~37°范围的XRD局部放大图; (c) LMFP31和(d) LMFP31-Ni的XRD精修图谱Figure 1 (a) XRD patterns and (b) local amplification of XRD at 34°~37° of LMFP31 and LMFP31-Ni. Rietveld refinement patterns of (c) LMFP31 and (d) LMFP31-Ni |
表1 LMFP31和LMFP31-Ni的XRD精修结果Table 1 The results of XRD refinement of LMFP31 and LMFP31-Ni |
| Sample | a/nm | b/nm | c/nm | V/nm3 |
|---|---|---|---|---|
| LMFP31 | 1.0429 | 0.6085 | 0.4741 | 0.3009 |
| LMFP31-Ni | 1.0422 | 0.6080 | 0.4737 | 0.3002 |
表2 Li—O键的键长变化Table 3 Changes in bond length of Li—O bonds |
| Sample | Bond length/nm | ||
|---|---|---|---|
| Li—O(1) | Li—O(2) | Li—O(3) | |
| LMFP31 | 0.2237 | 0.2118 | 0.2132 |
| LMFP31-Ni | 0.2238 | 0.2120 | 0.2136 |
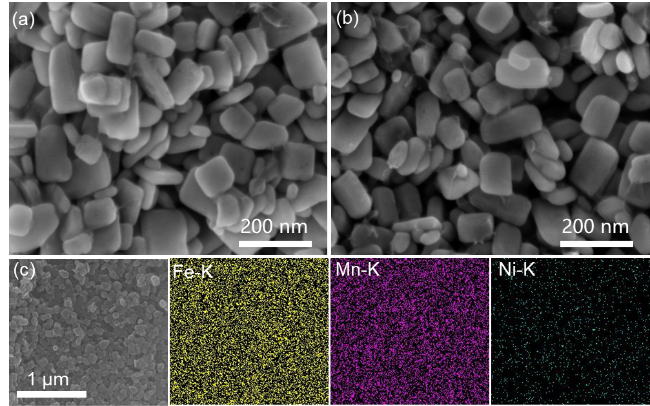
图3 (a) LMFP31和(b) LMFP31-Ni的SEM图, (c) LMFP31-Ni的元素面扫描图Figure 3 SEM images of (a) LMFP31 and (b) LMFP31-Ni, (c) EDS maps of LMFP31-Ni |
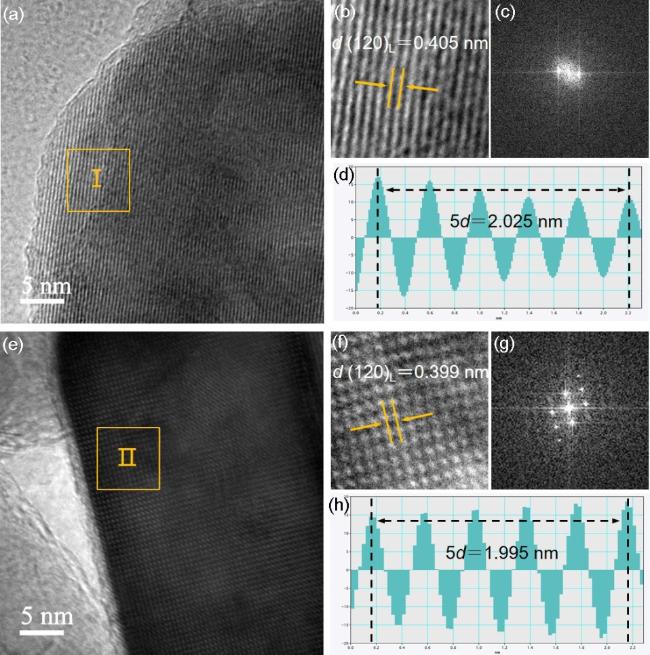
图4 LMFP31的微观结构分析: (a) HRTEM图, (b)区域Ⅰ的放大图, (c)区域Ⅰ的FFT图, (d) iFFT线性剖面图; LMFP31-Ni的微观结构分析: (e) HRTEM图, (f)区域Ⅱ的放大图, (g)区域Ⅱ的FFT图, (h) iFFT线性剖面图Figure 4 Microstructural analysis of LMFP31: (a) HRTEM image, (b) magnified view of area I, (c) FFT pattern of area I, (d) iFFT line profile; Microstructural analysis of LMFP31-Ni: (e) HRTEM image, (f) magnified view of area II, (g) FFT pattern of area II, (h) iFFT line profile |
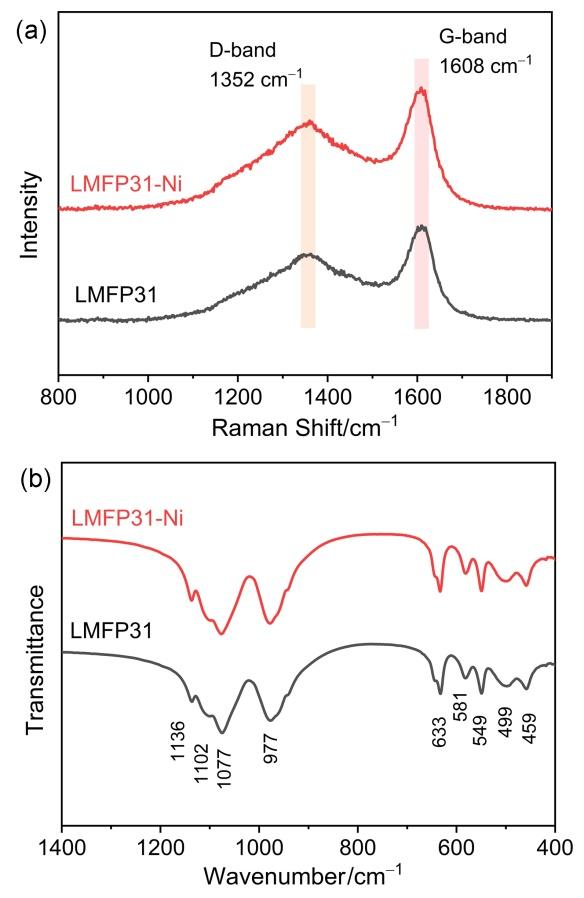
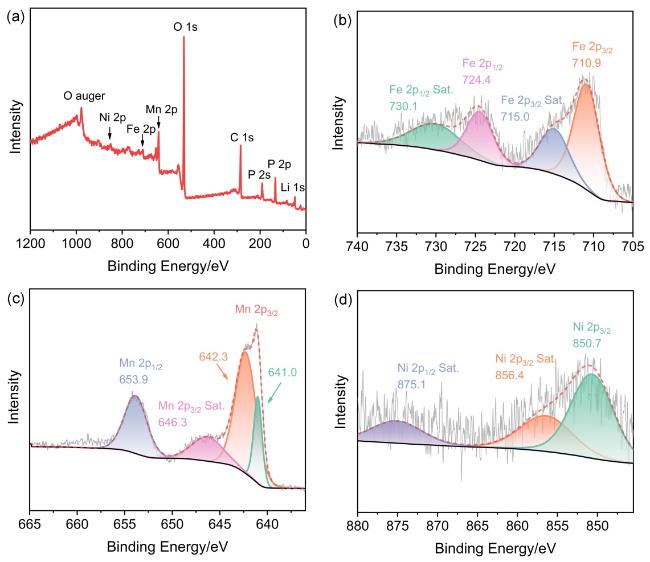
2.2 表面组分表征
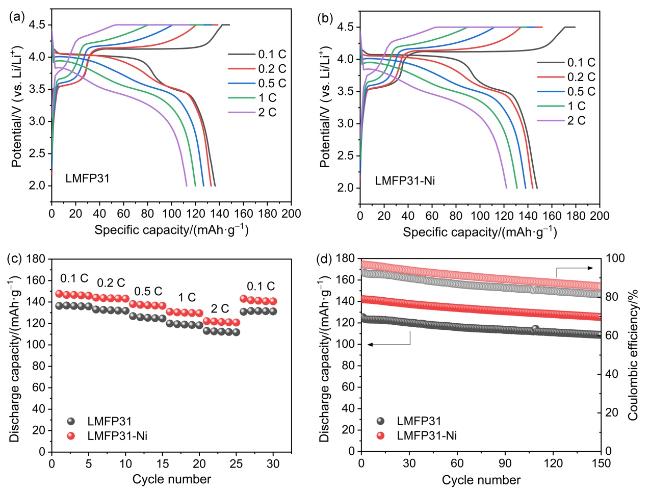
2.3 电化学性能分析
图6 LMFP半电池的电化学性能: (a) LMFP31和(b) LMFP31-Ni在不同倍率下的首圈充放电曲线; LMFP31和LMFP31-Ni的(c)倍率性能和(d)循环稳定性Figure 6 Electrochemical performance of LMFP half-cell: The first cycle charge and discharge curves at different rates of (a) LMFP31 and (b) LMFP31-Ni. (c) Rate performance and (d) cycling stability of LMFP31 and LMFP31-Ni |
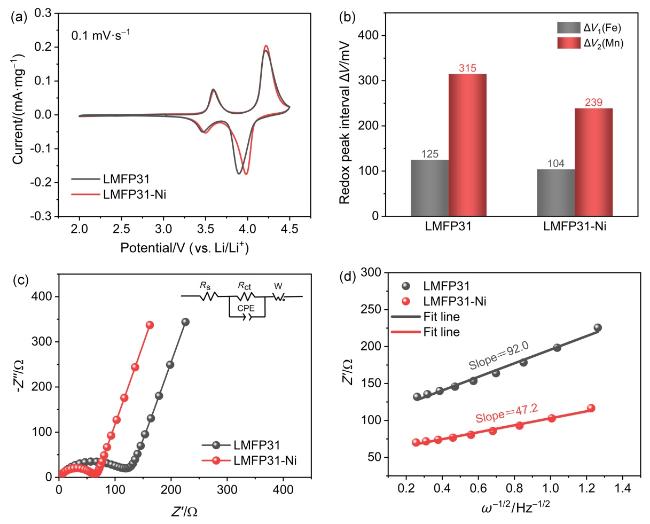
图7 LMFP半电池的动力学表征: (a) LMFP31和LMFP31-Ni半电池的CV曲线, (b)氧化还原峰间电压差, (c)等效电路和拟合后的奈奎斯特图, (d)低频区Z'与ω−1/2的线性拟合关系Figure 7 Kinetic characterization of LMFP half-cells: (a) CV curves of LMFP31 and LMFP31-Ni half-cell, (b) voltage difference between redox peak, (c) EIS spectra and the equivalent circuit, (d) the linear fitting relationship between Z' and ω−1/2 in the low frequency region of EIS |
表3 LMFP31和LMFP31-Ni的EIS测试结果Table 3 EIS test results of LMFP31 and LMFP31-Ni |
| Sample | Rct/Ω | DLi+/(×10−12 cm2•s−1) |
|---|---|---|
| LMFP31 | 128.1 | 3.3 |
| LMFP31-Ni | 57.3 | 5.2 |