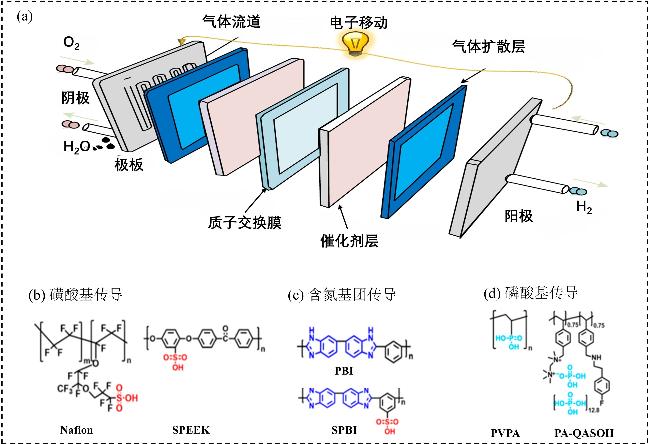
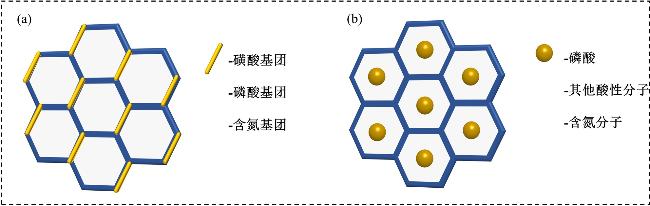
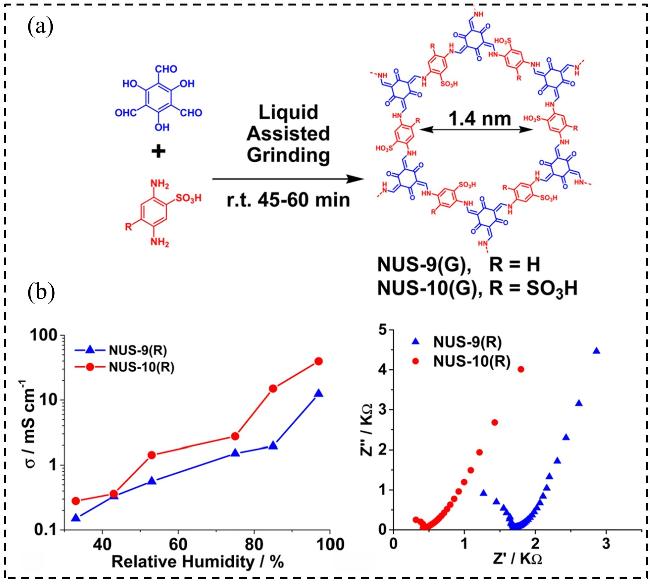
1 引言
2 COF基质子交换膜的制备和性能评测
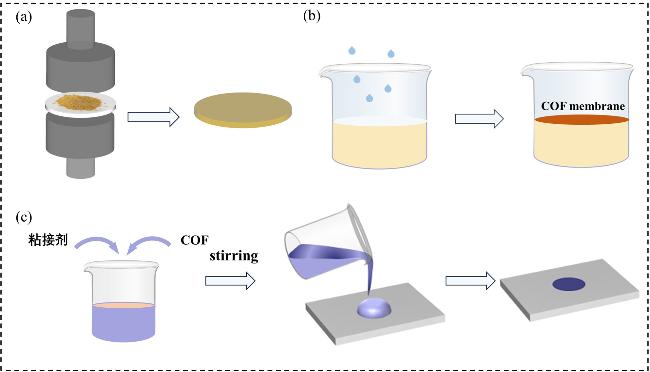
2.1 COF基质子交换膜制备
2.2 COF基质子交换膜的性能评测
2.2.1 质子传导率评测
2.2.2 质子传导活化能
2.2.3 离子交换容量评测(IEC)
2.2.4 机械性能评测
2.2.5 溶胀率测试
2.2.6 吸水率测试
2.3 膜电极(MEA)器件的组装评测
3 COF结构设计与质子传导性能研究
3.1 COF功能基团设计
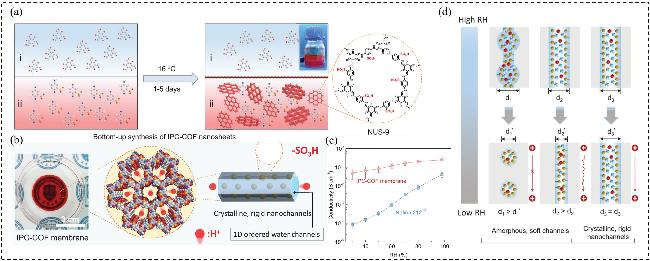
图5 (a) IPC-COF纳米片的自下而上合成示意图以及NUS-9的化学结构; (b) IPC-COF膜组装及孔结构示意图; (c) 40 ℃时IPC-COF膜和Nafion 212的质子传导率随RH的变化; (d)不同类型PEMs的离子传输通道随相对湿度降低的变化示意图Figure 5 (a) Schematic illustration of the bottom-up synthesis of IPC-COF nanosheets and the chemical structure of NUS-9; (b) Schematic illustration of IPC-COF membrane assembly and pore structures; (c) Proton conductivity of IPC-COF membrane and Nafion 212 versus RH at 40 ℃; (d) Schematic illustration of ion transport channels changes with decreased RH for different types PEMs[38]. Copyright © 2020 Wiley- VCH GmbH |
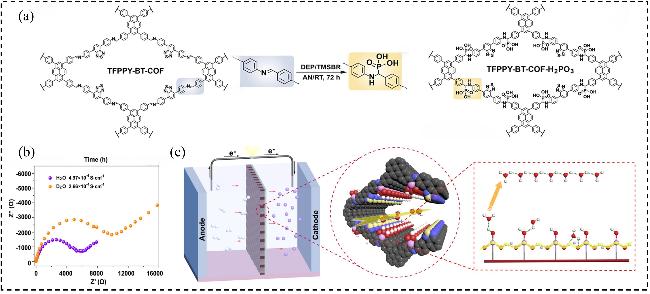
图7 (a) TFPPY-BT-COF-H2PO3 不对称氢膦酰化反应的化学结构演变; (b) TFPPY-BT-COF-H2PO3在H2O和D2O中(313 K, RH=98%)测得的Nyquist图; (c) TFPPY-BT-COF-H2PO3的模拟质子传导机制示意图Figure 7 (a) Chemical structure evolution of the asymmetric hydrophosphonylation of TFPPY-BT-COF-H2PO3; (b) Nyquist plots of TFPPY- BT-COF-H2PO3 measured with H2O and D2O (313 K, 98% RH); (c) Illustration for the proposed proton conduction mechanism of TFPPY- BT-COF-H2PO3[55]. Copyright © 2022, American Chemical Society |
3.2 COF孔径调控
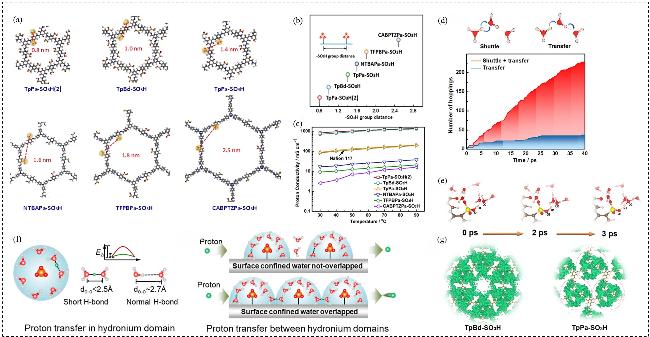
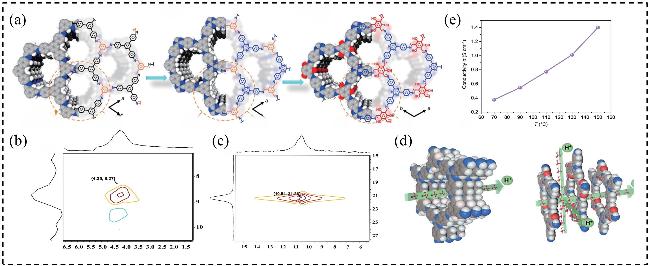
图8 (a) iCOF拓扑结构的分子模拟; (b) iCOFs骨架中-SO3H基团距离示意图; (c) 在RH=100%条件下, 膜的质子电导率随温度的变化情况; (d) iCOFMs中的质子转移机制: “穿梭”和“转移”示意图, 以及质子跳跃次数随模拟时间的变化情况; (e)质子的易解离行为; (f)质子在水合氢离子域内和域之间的转移; (g)水合氢离子(H3O+)在TpBd-SO3H和TpPa-SO3H中的概率密度分布Figure 8 (a) The molecule simulation of the iCOF topological structure; (b) Scheme of the -SO3H group distance in iCOFs skeleton; (c) Temperature dependent proton conductivities of the membranes under RH=100%; (d) Proton transfer mechanism in iCOFMs, schematics of a shuttle and a transfer, and the number of proton hopping as a function of the simulation time; (e) Facile proton dissociation behavior; (f) proton transfer in and between hydronium domain; (g) The probability density distribution of H3O+ in TpBd-SO3H and TpPa-SO3H[56] |
4 COF孔内限域质子载体分子
4.1 COF限域磷酸分子的界面调控
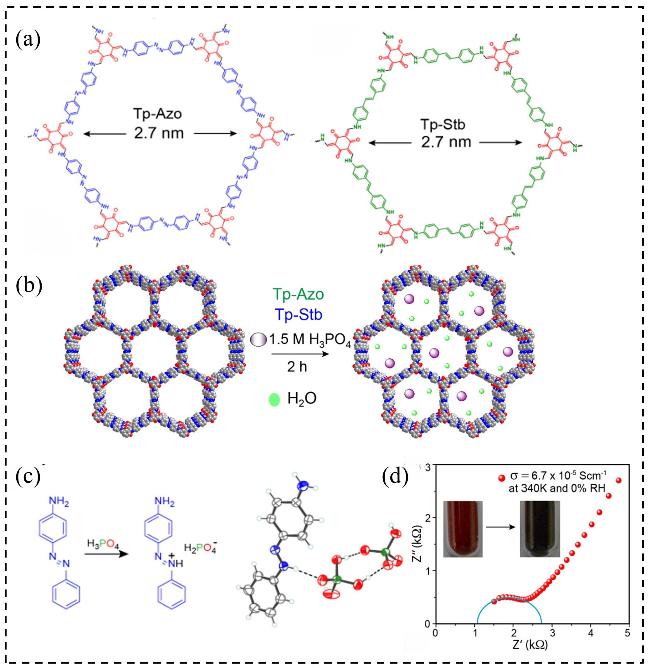
图9 (a) Tp-Azo和Tp-Stb的合成示意图; (b) COFs中H3PO4掺杂的示意图; (c) 4-[(E)-苯基偶氮基]苯胺二氢磷酸盐的晶体结构; (d)磷酸负载的Tp-Azo(PA@Tp-Azo)在无水条件下的质子传导率Figure 9 (a) Schematic representation of synthesis of Tp-Azo and Tp-Stb; (b) Schematic representation of H3PO4 doping in COFs; (c) Crystal structure of 4-[(E)-phenyl-diazenyl]anilinium dihydrogen phosphate; (d) Proton conductivity of PA@Tp-Azo in anhydrous condition[67] |
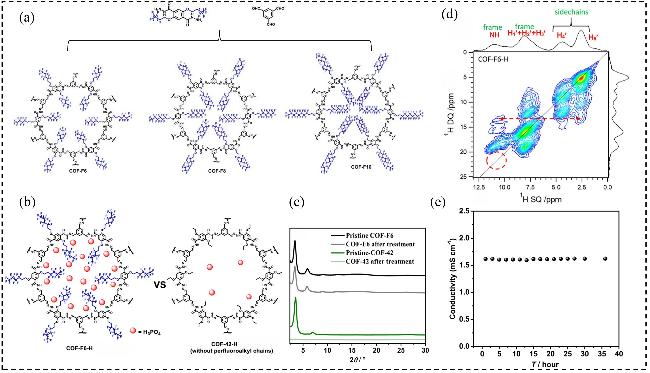
图10 (a)自下而上合成COF-Fx (x=6、8、10)的策略; (b)在无水条件下, 负载42% (w) H3PO4的COF-F6-H和COF-42-H的示意图; (c) COF-F6和COF-42在磷酸浸泡处理前后的PXRD图谱; (d) COF-F6-H的二维1H/1D图谱; (e) COF-F6-H的质子传导率长周期测试Figure 10 (a) A bottom-up strategy for the synthesis of COF-Fx (x=6, 8, 10); (b) Scheme of COF-F6-H and COF-42-H with 42% (w) H3PO4 loading under anhydrous conditions; (c) PXRD patterns of COF-F6 and COF-42 before and after H3PO4 soaking treatments; (d) 2D 1H/1D spectra for COF-F6-H; (e) Long-period test for COF-F6-H[69]. Copyright © 2020, American Chemical Society |
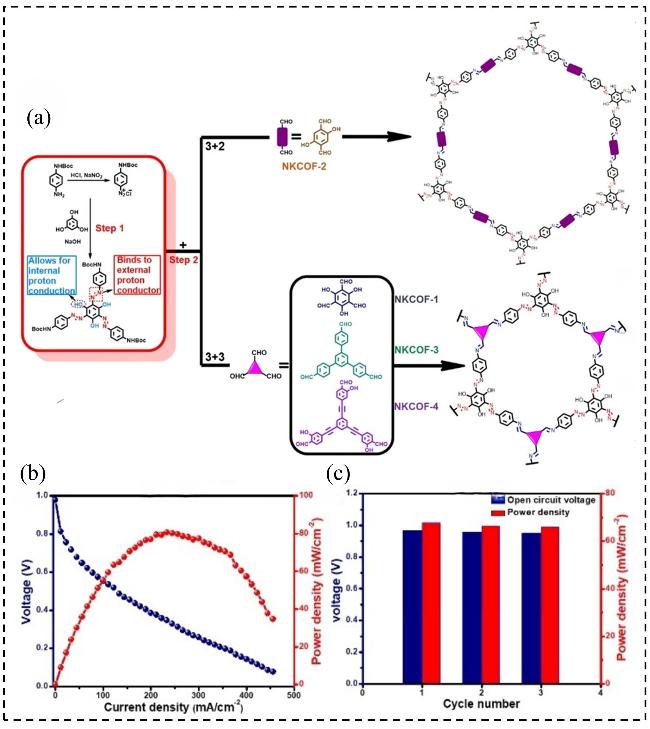
图11 (a) NKCOFs的合成路线示意图; (b)在RH=100%, T=60 ℃的条件下, 使用H2/O2单电池组件测得H3PO4@NKCOF-1的燃料电池极化曲线和功率密度曲线; (c) H3PO4@NKCOF-1的OCV和功率密度的循环稳定性测试Figure 11 (a) Illustration of the synthetic route of NKCOFs; (b) Fuel cell polarization curves and power density curves of H3PO4@NKCOF-1 measured at 60 ℃ using single H2/O2 cell assembly under 100% RH; (c) Cyclic stability of OCV and power density of H3PO4@NKCOF-1[60]. Copyright © 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim |
4.2 COF限域磷酸分子的孔结构调控
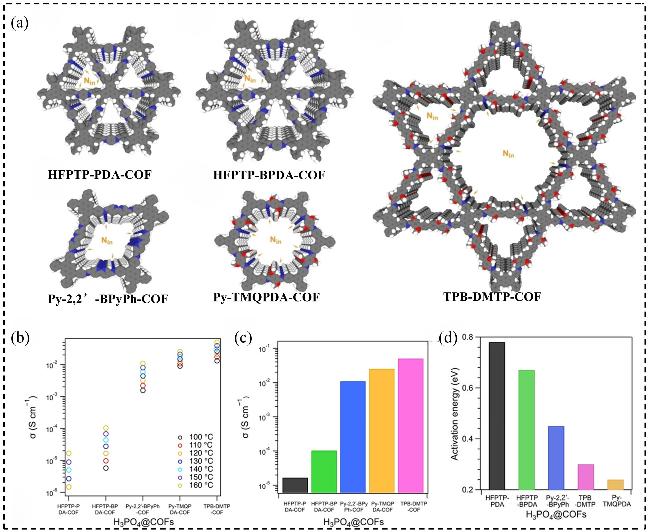
图12 (a) HFPTP-PDA-COF、HFPTP-BPDA-COF、Py-2,2-BPyPh- COF、TPB-DMTP-COF及Py-TMQPDA-COF的重构单孔结构; (b) H3PO4@COFs材料在不同温度下的无水质子传导率; (c) 160 ℃时H3PO4@COFs的无水质子传导率; (d) H3PO4@COFs的活化能垒Figure 12 (a) Reconstructed one pore structures of HFPTP-PDA-COF, HFPTP-BPDA-COF, Py-2,2-BPyPh-COF, TPB-DMTP-COF, and Py-TMQPDA-COF; (b) Anhydrous proton conductivity of H3PO4@COFs at different temperatures; (c) Anhydrous proton conductivity of H3PO4@COFs at 160 ℃; (d) Activation energy barriers of H3PO4@COFs[72]. Copyright © 2024 The Author(s). Angewandte Chemie International Edition published by Wiley-VCH GmbH |
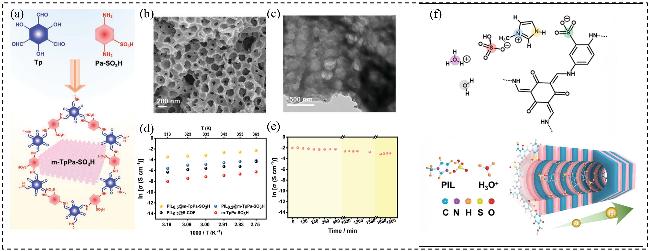
图13 (a)分级宏-微孔结构m-TpPa-SO3H的合成过程示意图; (b, c)分级结构m-TpPa-SO3H的SEM图和TEM图; (d) m-TpPa-SO₃H、PIL0.2@m-TpPa-SO₃H、PIL0.5@m-TpPa-SO₃H及PIL0.2@s-COF质子传导率随温度变化曲线; (e) PIL0.5@m-TpPa-SO₃H在90 ℃、RH=100%下的质子传导率长期稳定性; (f)在RH=100% 条件下, PIL0.5@m-TpPa-SO3H的质子传导机制示意图Figure 13 (a) Schematic illustration of the synthesis process of hierarchical macro-microporous m-TpPa-SO3H; (b, c) The SEM images and TEM image of hierarchical m-TpPa-SO3H; (d) Temperature dependence of the proton conductivities of m-TpPa-SO3H, PIL0.2@m-TpPa-SO3H, PIL0.5@m-TpPa-SO3H, and PIL0.2@s-COF; (e) Long-term proton conductivity durability of PIL0.5@m-TpPa-SO3H at 90 ℃ under RH=100%; (f) Schematic illustration of proton conduction mechanism of PIL0.5@m-TpPa-SO3H under RH=100%[73]. Copyright © 2023 Wiley-VCH GmbH |
图14 (a)孔径相近但亲水基团数量不同的COF-1、COF-2和COF-3的结构; (b, c) COF-3的二维1H DQ/SQ核磁共振光谱; (d)质子在一维和三维方向上的传导路径示意图; (e) COF-3@PA-30的质子电导率随温度的变化情况Figure 14 (a) The structure of COF-1, COF-2 and COF-3 with similar pore diameters but different amounts of hydrophilic groups; (b, c) 2D 1H DQ/SQ NMR spectrum of COF-3; (d) Proton conduction pathway schematic illustration in 1D and 3D direction; (e) The temperature-dependent proton conductivity of COF-3@PA-30[31]. Copyright © 2023, Tsinghua University Press |
图15 (a)三维COF NUST-28的合成路径; (b) NUST-28与磷酸之间的氢键连接; (c) NUST-28-60%的质子传导长期稳定性Figure 15 (a) Illustration for the synthesis of NUST-28; (b) The hydrogen bonding between NUST-28 and H3PO4; (c) Long-term proton conduction of NUST-28-60%[64] |
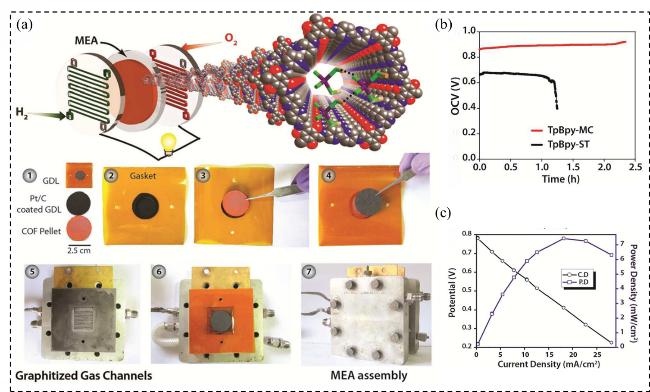
图16 (a)使用COF粉末制作MEA的示意图: (1)制备涂有Pt的气体扩散层(GDL, 用作阳极和阴极)、COF压片(用作固体电解质), (2~4)将COF压片夹在两个电极之间, (5~7)将膜电极组件组装成单电池堆; (b)在PEMFC中, 使用H3PO4负载的PA@TpBpy-ST和PA@TpBpy-MC作为固体电解质测得的OCV寿命数据; (c)在50 ˚C下, 使用干燥H2、Pt、C/COF压片/Pt、C、干燥O2的电化学电池测得的燃料电池极化图Figure 16 (a) Schematic representation of MEA making using pelletized COF powder: (1) Preparation of Pt coated GDL (as anode and cathode), COF pellet (as solid electrolyte), (2~4) Sandwiching of COF pellet between two electrodes, (5~7) Assembly of the MEA into a single cell stack; (b) Lifetime OCV measurement obtained using PA@TpBpy-ST and PA@TpBpy-MC as solid electrolytes in PEMFC; (c) Fuel cell polarization plot obtained at 50 ℃ using dry H2, Pt, C/COF pellet/Pt, C, dry O2 electrochemical cell[47] |
4.3 COF限域其他客体分子
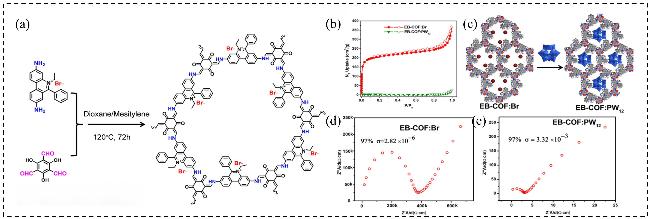
图17 (a) EB-COF:Br的合成示意图; (b) EB-COF:Br和EB-COF:PW12的氮气吸附等温线; (c) PW12O40³⁻限域COF的示意图; (d)在RH=97%条件下EB-COF:Br和(e) EB-COF:PW12的质子传导率Figure 17 (a) Schematic of the synthesis of EB-COF:Br; (b) Nitrogen sorption isotherms for EB-COF:Br and EB-COF:PW12; (c) Schematic of PW12O403- doping in COF; (d) Proton conductivity of EB-COF:Br and (e) EB-COF:PW12 in RH=97% condition[77]. Copyright © 2016, American Chemical Society |
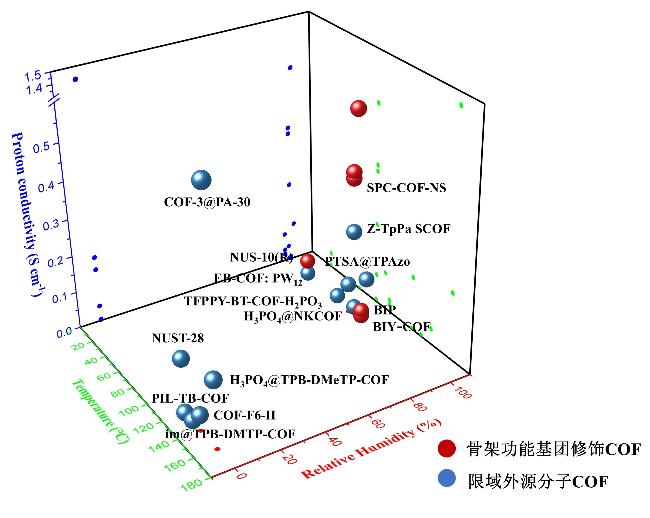
表1 负载不同客体分子的COFs材料的质子传导性能Table 1 The proton conduction properties of COFs materials loaded with different sources |
| COFs | Sources of conductivity | Proton conductivity/(S•cm−1) | T/℃ | RH/% |
|---|---|---|---|---|
| TFPPY-BT-COF-H2PO3[65] | H3PO4 | 1.12×10−3 | 60 | 98 |
| NUST-28[74] | H3PO4 | 1.46×10−1 | 120 | 0 |
| COF-3@PA-30[31] | H3PO4 | 1.4 | 160 | 0 |
| H3PO4@TPB-DMeTPCOF[68] | H3PO4 | 1.91×10−1 | 160 | 0 |
| COF-F6-H[69] | H3PO4 | 4.2×10−2 | 140 | 0 |
| H3PO4@Tp-Azo-COF[67] | H3PO4 | 6.70×10−5 | 67 | 0 |
| H3PO4@NKCOFs[70] | H3PO4 | 1.13×10−1 | 80 | 98 |
| H3PO4@TpBpy-MC[47] | H3PO4 | 2.50×10−3 | 120 | 0 |
| Phytic acid@TpPa- (SO3H-Py)[75] | Phytic acid | 5.00×10−4 | 120 | 0 |
| PTSA@TpAzo[43] | p-Toluenesulfonic acid | 7.8×10−2 | 80 | 95 |
| EB-COF:PW12[77] | polyoxometalates | 3.32×10−3 | 20 | 97 |
| trz@TPB-DMTP-COF[58] | Triazole | 1.1×10−3 | 130 | 0 |