1 引言
2 结果与讨论
2.1 单晶Co(SCN)2(pyz)2的表征
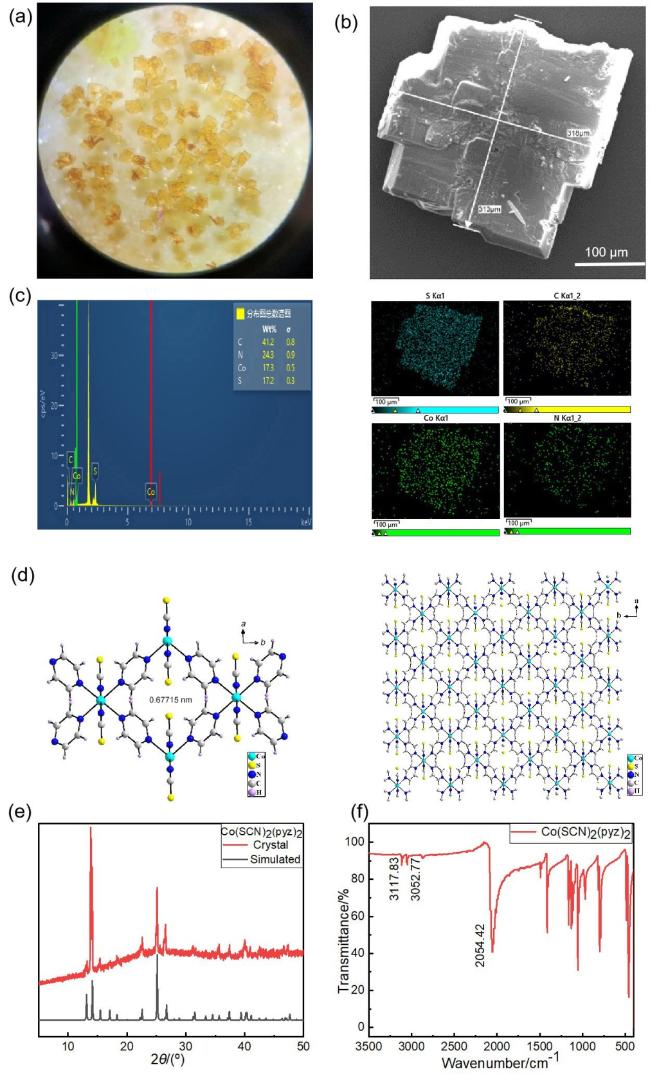
图1 (a) MOF Co(SCN)2(pyz)2的光学图片; (b) MOF Co(SCN)2(pyz)2的扫描电镜图; (c) MOF Co(SCN)2(pyz)2的元素分析图; (d) Co(SCN)2(pyz)2单晶的结构图, 以及延c轴方向的二维平面结构; (e) MOF Co(SCN)2(pyz)2的粉末X射线衍射图; (f) MOF Co(SCN)2(pyz)2的红外光谱图Figure 1 (a) Optical photograph of MOF Co(SCN)2(pyz)2; (b) SEM image of MOF Co(SCN)2(pyz)2; (c) EDS mapping of Co(SCN)2(pyz)2; (d) Structural diagram of Co(SCN)2(pyz)2 single crystal and two-dimensional planar structure along the c-axis direction; (e) XRD pattern of Co(SCN)2(pyz)2; (f) IR spectra of Co(SCN)2(pyz)2 |
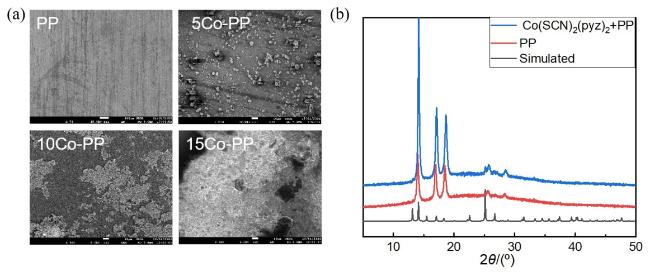
2.2 Co(SCN)2(pyz)2@PP复合隔膜的表征
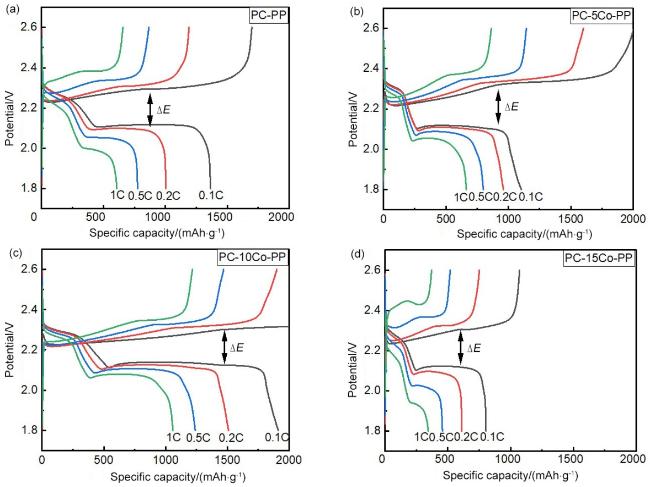
2.3 Co(SCN)2(pyz)2@PP复合隔膜组装的锂硫电池的性能研究
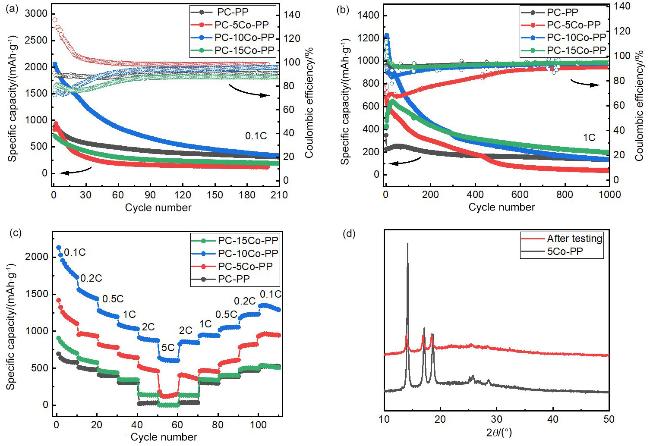
图4 (a) 0.1 C倍率下, 四种锂硫电池的长循环性能测试; (b) 1 C倍率下, 四种锂硫电池的长循环性能测试; (c) 4种锂硫电池的倍率性能; (d) PC- 5Co-PP锂硫电池1 C倍率下长循环测试后的复合隔膜5Co-PP的粉末X射线衍射Figure 4 (a) Long cycle performance testing of four types of lithium sulfur batteries at a 0.1 C rate; (b) Long cycle performance testing of four types of lithium sulfur batteries at 1 C rate; (c) Rate performance of four types of lithium sulfur batteries; (d) Powder X-ray diffraction of composite separator 5Co-PP after long cycle testing at 1 C rate for PC-5Co-PP lithium sulfur battery |