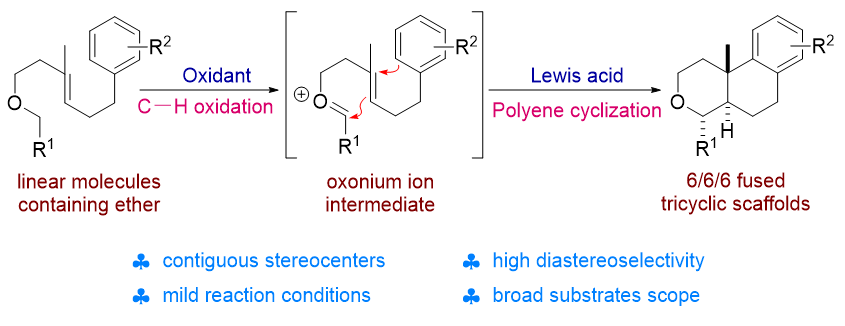
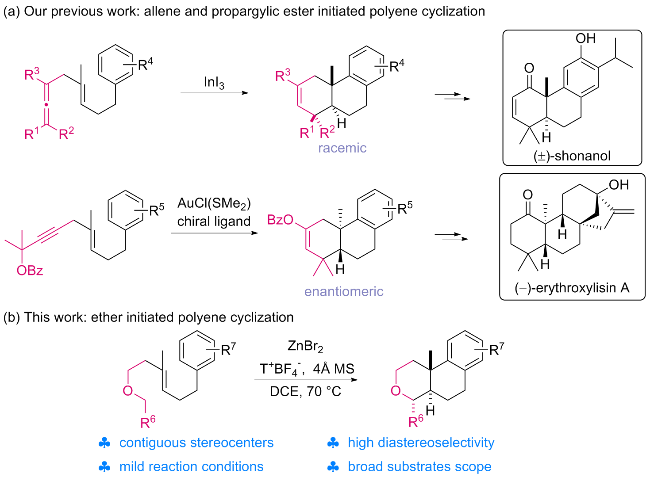
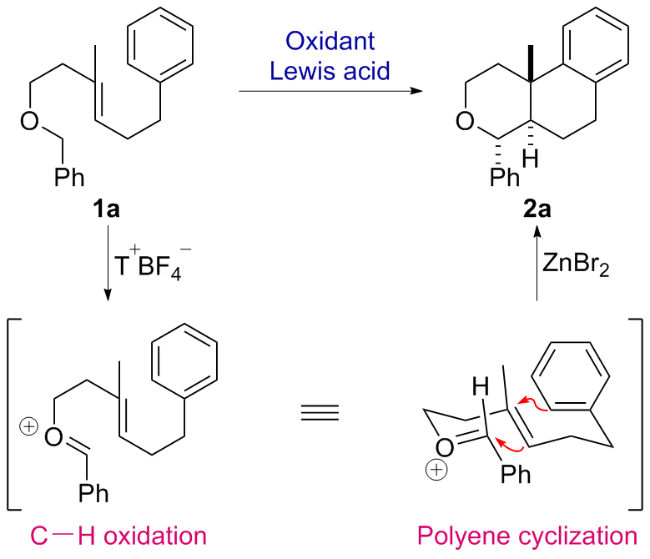
1 引言
2 结果与讨论
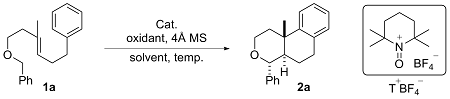
2.1 反应条件优化
表1 反应条件筛选Table 1 Screening of reaction conditions |
| Entry | Lewis acid | Oxidant | Solvent | Temp./℃ | Yield/% | dr |
|---|---|---|---|---|---|---|
| 1 | InCl3 | T+BF4− | DCE | 70 | 88 | >20∶1 |
| 2 | Cu(OAc)2 | T+BF4− | DCE | 70 | 85 | >20∶1 |
| 3 | ZnCl2 | T+BF4− | DCE | 70 | 81 | >20∶1 |
| 4 | ZnBr2 | T+BF4− | DCE | 70 | 92 | >20∶1 |
| 5 | ZnBr2 | CAN | DCE | 70 | n.d. | — |
| 6 | ZnBr2 | TBHP | DCE | 70 | n.d. | — |
| 7 | ZnBr2 | DTBP | DCE | 70 | n.d. | — |
| 8 | ZnBr2 | Chloranil | DCE | 70 | n.d. | — |
| 9 | ZnBr2 | K2S2O8 | DCE | 70 | n.d. | — |
| 10 | ZnBr2 | T+BF4− | CH2Cl2 | 70 | 70 | >20∶1 |
| 11 | ZnBr2 | T+BF4− | CHCl3 | 70 | 44 | >20∶1 |
| 12 | ZnBr2 | T+BF4− | Toluene | 70 | n.d. | — |
| 13 | ZnBr2 | T+BF4− | MeCN | 70 | n.d. | — |
| 14 | ZnBr2 | T+BF4− | DMF | 70 | n.d. | — |
| 15 | ZnBr2 | T+BF4− | 1,4-Dioxane | 70 | n.d. | — |
| 16 | ZnBr2 | T+BF4− | DCE | r.t. | n.d. | — |
| 17 | ZnBr2 | T+BF4− | DCE | 50 | 55 | >20∶1 |
| 18 | ZnBr2 | T+BF4− | DCE | 90 | 85 | >20∶1 |
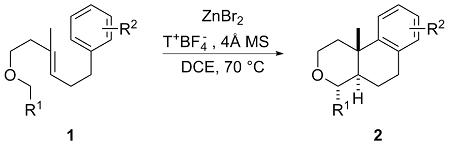
2.2 底物普适性考察
表2 底物适用性考察Table 2 Substrate scope investigation |
 |
aThe reactions were conducted with 1 (0.15 mmol), T+BF4− (2.0 equiv.), ZnBr2 (10 mol%) and 4Å activated molecular sieve (30 mg) in DCE (1.0 mL) at 70 ℃ for 4 h. Isolated yield. dr determined by 1H NMR. |