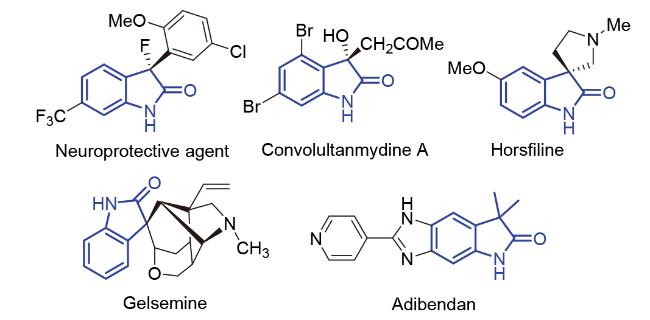
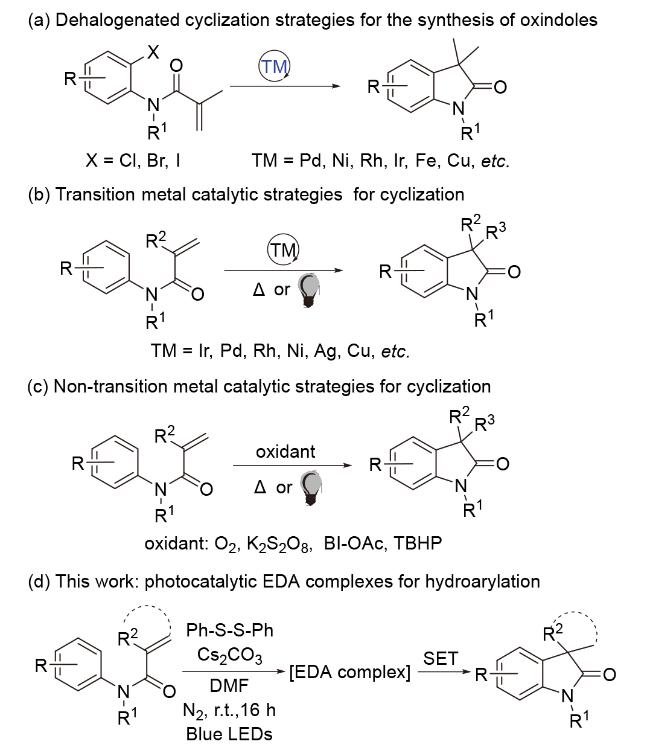
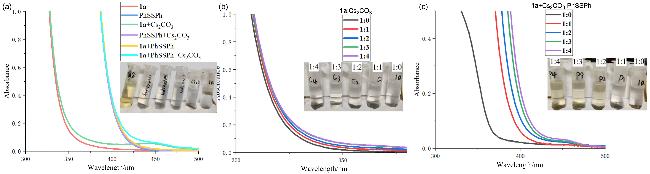
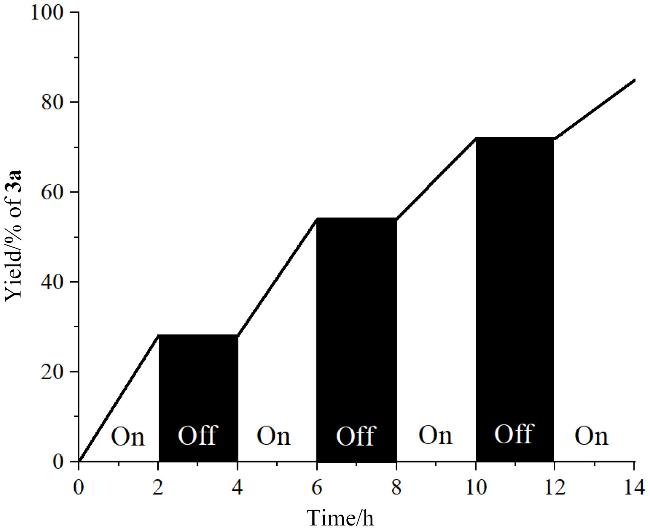
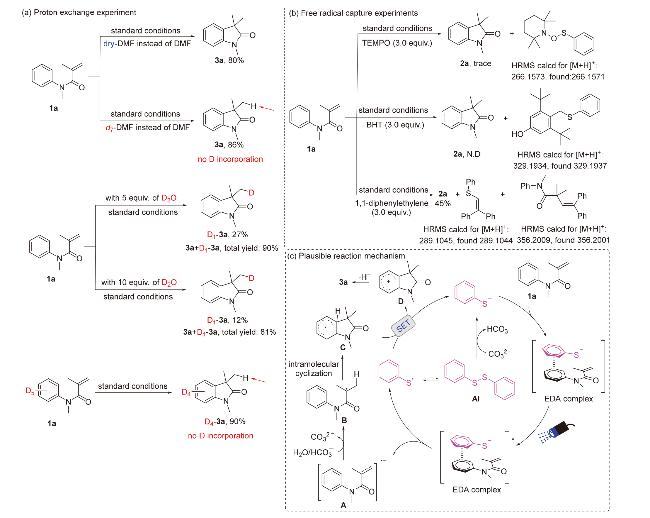
1 引言
2 结果与讨论
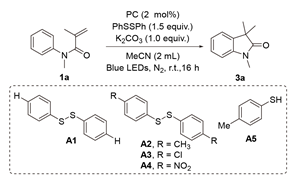
2.1 反应条件优化
表1 反应条件优化Table 1 Conditions optimization |
| Entry | Variations from standard conditions | Yieldb/% |
|---|---|---|
| 1 | none | 56 |
| 2 | A2 instead of A1 | trace |
| 3 | A3 instead of A1 | 35 |
| 4 | A4 instead of A1 | n.r. |
| 5 | A5 instead of A1 | 25 |
| 6 | Cs2CO3 instead of K2CO3 | 70 |
| 7 | DABCO instead of K2CO3 | trace |
| 8 | DBU instead of K2CO3 | 45 |
| 9 | DIPEA instead of K2CO3 | 35 |
| 10 | H2O instead of MeCN | n.r. |
| 11 | DMF instead of MeCN | 90 |
| 12 | DMSO instead of MeCN | trace |
| 13 | 390~395 nm or white light | n.r. |
| 14 | air instead of N2 | 55 |
| 15 | O2 instead of N2 | n.d |
| 16 | without A1 or Cs2CO3 or light irradiation | n.r. |
a Reaction conditions: 1a (0.2 mmol), sulfur anion precursors (0.3 mmol) and base (0.2 mmol) in solvent (2 mL) at room temperature for 16 h under N2, 18 W blue LED (λ=455~460 nm). b Isolated yields. |
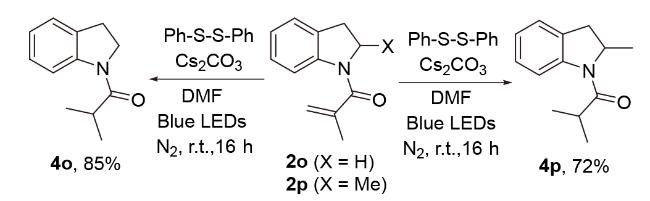
2.2 底物拓展
表2 光催化N-芳基酰胺的底物范围Table 2 Substrate scope of photocyclization of N-arylacrylamides |
| |
a Reaction conditions: N-arylacrylamides (0.2 mmol), PhSSPh (0.3 mmol), and Cs2CO3 (0.2 mmol) in DMF (2 mL) at room temperature for 16 h under N2, 18 W blue LED (λ=455~460 nm). b Isolated yields. |