1 引言
2 结果与讨论
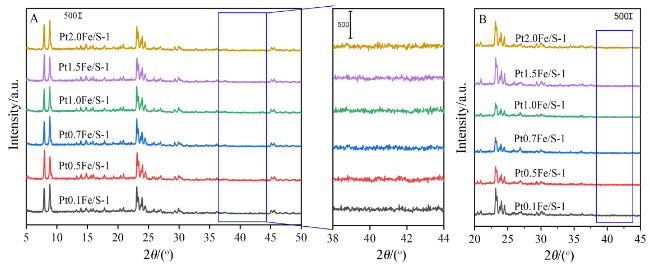
2.1 X射线衍射(XRD)表征结果及分析
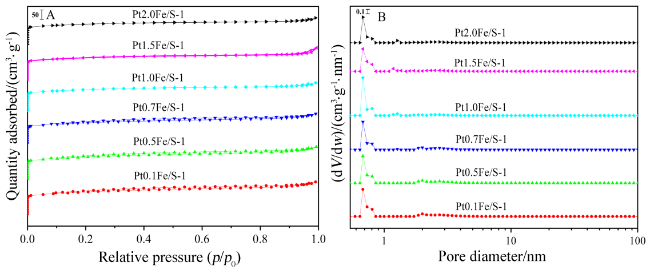
2.2 N2-吸附脱附表征结果及分析
图2 焙烧后PtFe/S-1催化剂的(A) N2-吸附脱附等温线及(B)孔径分布图Figure 2 (A) N2 adsorption-desorption isotherms and (B) pore size distribution of the calcined PtFe/S-1 catalysts |
表1 PtFe/S-1催化剂的织构性能参数Table 1 Textural properties of PtFe/S-1 catalysts |
| Sample | Smica/(m2•g⁻1) | SBETb/(m2•g⁻1) | davec/nm | dBJHd/nm | Vmice/(cm3•g⁻1) | Vt f/(cm3•g⁻1) |
|---|---|---|---|---|---|---|
| Pt0.1Fe/S-1 | 221.5 | 434.5 | 2.4 | 4.4 | 0.11 | 0.26 |
| Pt0.5Fe/S-1 | 208.4 | 423.5 | 2.4 | 4.5 | 0.11 | 0.25 |
| Pt0.7Fe/S-1 | 206.0 | 407.3 | 2.4 | 4.5 | 0.11 | 0.24 |
| Pt1.0Fe/S-1 | 251.5 | 376.3 | 2.3 | 4.4 | 0.13 | 0.22 |
| Pt1.5Fe/S-1 | 260.3 | 392.5 | 2.3 | 4.5 | 0.13 | 0.23 |
| Pt2.0Fe/S-1 | 248.4 | 366.9 | 2.3 | 4.6 | 0.13 | 0.21 |
a Calculated by the t-Plot method; b BET Surface area; c Average pore diameter (4V/A by BET); d Average pore diameter was calculated using the BJH method; e t-Plot micropore volume; f Single point adsorption total pore volume of pores with diameter<40.31 nm at p/p0=0.95. |
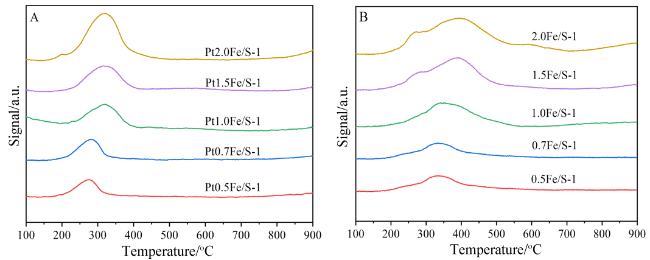
2.3 氢气程序升温还原(H2-TPR)表征结果及分析
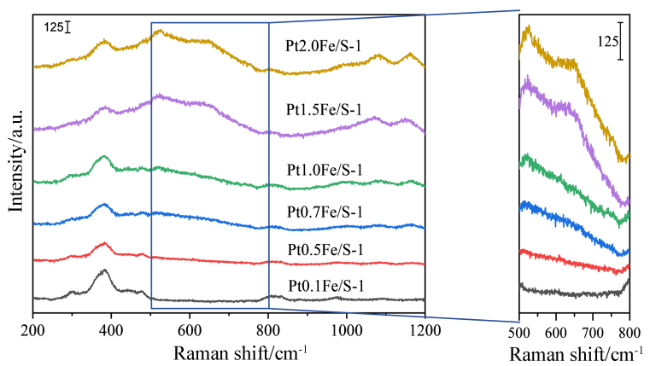
2.4 Raman表征结果及分析
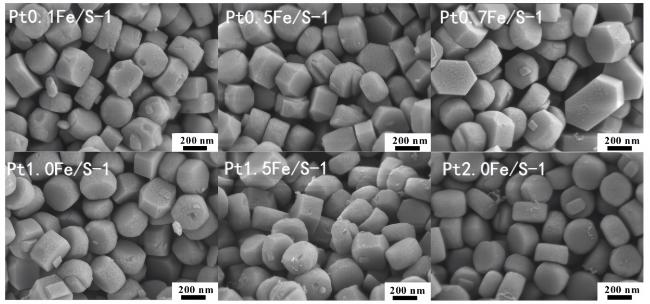
2.5 扫描电子显微镜(SEM)表征结果及分析
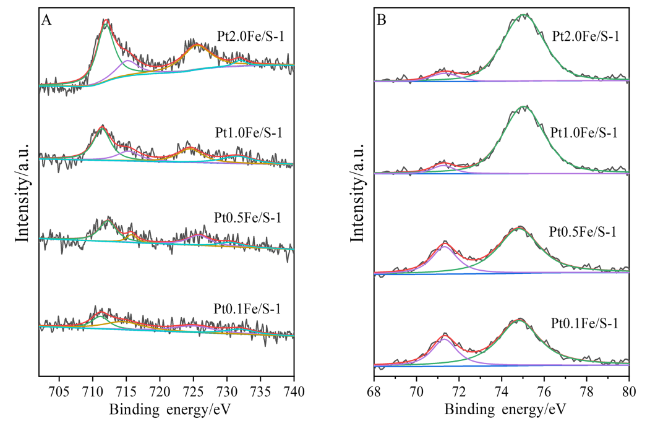
2.6 X射线光电子能谱(XPS)表征结果及分析
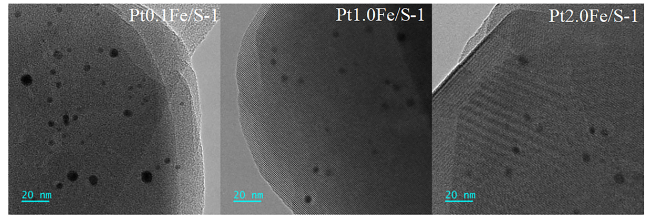
2.7 透射电子显微镜(TEM)表征结果及分析
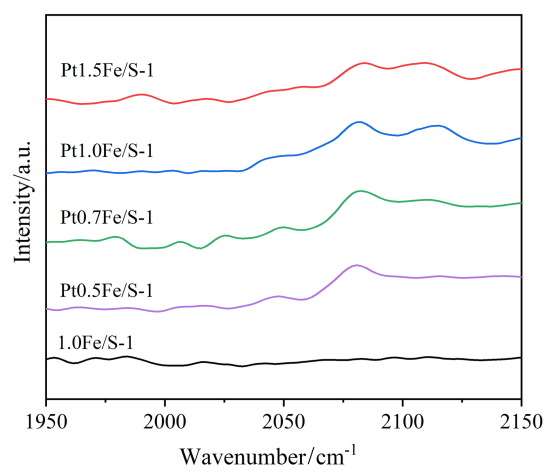
2.8 一氧化碳漫反射傅里叶变换红外光谱(CO-DRIFT)表征结果及分析
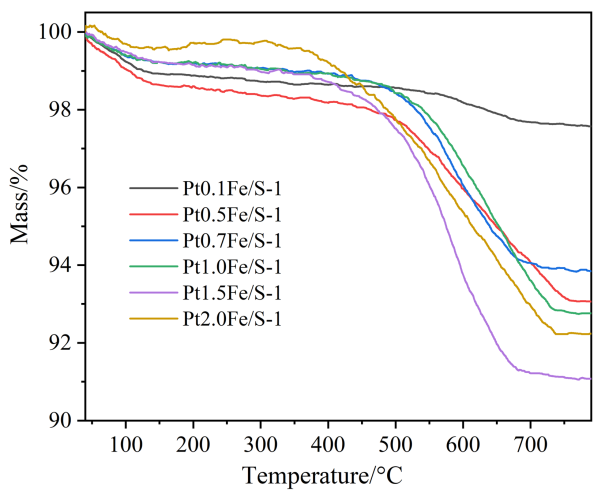
2.9 PtFe/S-1催化剂的丙烷脱氢活性评价结果及分析
图9 1.0Fe/S-1和PtFe/S-1催化剂上(A)丙烷转化率和(B)丙烯选择性随时间变化关系图; (C) PtFe/S-1催化剂的活化能Figure 9 The relationship between (A) propane conversion and (B) propylene selectivity over time during propane dehydrogenation over 1.0Fe/S-1 and PtFe/S-1 catalysts; (C) Activation energy of PtFe/S-1 catalysts |
表2 PtFe/S-1和1.0Fe/S-1催化剂的丙烷脱氢性能Table 2 Propane dehydrogenation performance of PtFe/S-1 and 1.0Fe/S-1 catalysts |
| Sample | Propane conversion/% | Propylene selectivity/% | kd/h⁻1 | |||
|---|---|---|---|---|---|---|
| Initial | Final | Initial | Final | |||
| Pt0.1Fe/S-1 Pt0.5Fe/S-1 Pt0.7Fe/S-1 Pt1.0Fe/S-1 Pt1.5Fe/S-1 Pt2.0Fe/S-1 1.0Fe/S-1 | 20.9 48.5 55.7 59.8 59.0 59.9 3.5 | 2.9 12.6 35.1 52.9 55.3 56.2 3.0 | 92.6 93.6 93.7 89.1 89.4 85.6 73.2 | 59.2 94.3 96.9 96.4 95.1 94.7 73.0 | 0.363 0.313 0.141 0.046 0.025 0.025 0.027 | |